1. The PIAS Proteins
The acronym PIAS stands for Protein Inhibitors of Activated STATs, a name derived from the foundational role these proteins play in negatively regulating Signal Transducers and Activators of Transcription (STAT) signaling pathways [1, 2]. Initially discovered using the yeast two-hybrid (Y2H) system, PIAS proteins are evolutionarily conserved across eukaryotes, from yeast to mammals. These versatile cellular regulators are known to interact with and modulate the function of approximately 60 different proteins [3, 4].
The PIAS family in mammals consists of four genes, which through alternative splicing, generate seven distinct proteins: PIAS1, PIASxα, PIASxβ, PIAS3, PIAS3L, PIASy, and PIASyE6 (also known as PIAS4). These proteins are ubiquitously expressed across eukaryotic and mammalian cells 19526197. Within eukaryotic cells, PIAS proteins are critical in modulating a diverse array of cellular processes. These include nuclear transport, regulation of Sma- and Mad-related protein (SMAD) signaling, and DNA damage repair, primarily through the recruitment and modulation of various transcription factors [5].
2. Historical Perspective
PIAS3 was the first member of the family to be identified in 1997. It was characterized as an inhibitor of interferon-alpha (IFN-α)-induced STAT3-mediated transcriptional regulation, operating within both cytoplasmic and nuclear compartments of human and mouse cell lines [6]. A year later, in 1998, four additional PIAS family members were isolated from JY112 B cells. Co-immunoprecipitation studies revealed that PIAS1 specifically interacts with STAT1, masking its DNA binding domain, but showed no interaction with STAT2 or STAT3 [3].
Shortly after these initial discoveries, the PIAS family was recognized for its broader role in negatively regulating the JAK/STAT pathway, effectively inhibiting signal transduction cascades [7]. The co-modulatory expression of PIAS1 in monkey and mouse systems was first reported in this period. Notably, the co-expression of PIAS1 with androgen receptors (ARs) was found to be essential for AR-mediated initiation and maintenance of spermatogenesis [8]. PIAS3 was also found to interact with ARs, acting as a co-regulator in prostate cancer contexts [9]. In 2001, PIASy was identified as having a distinct function as a blocker of AR activity in prostate cancer [10].
A landmark discovery in 2001 highlighted the role of PIAS proteins in catalyzing the SUMOylation of various proteins, including LEF1, p53, and STAT proteins [11]. The “PINIT” domain, a highly conserved 181 amino acid domain within the PIAS family, was identified as crucial for the nuclear localization of PIAS3L in mice [12]. PIASy was further shown to interact with Smad proteins and function as a downregulator of Smad-mediated transcriptional responses by recruiting histone deacetylase 1 (HDAC1) [13]. Interestingly, further research determined that PIAS3 could also stimulate Smad transcriptional activity [13], highlighting the complex and sometimes contradictory roles of PIAS family members.
Progress in PIAS research then shifted towards exploring their potential as anticancer therapeutic targets. Overexpression of PIAS3 was shown to reduce STAT3 transcription in glioblastoma and ovarian cancer. Curcumin was the first natural compound reported to control activated STAT3 by modulating the expression of PIAS and JAK/STAT suppressor genes in cancer [14].
Further investigations explored the E3-SUMO ligase activity of PIAS4 as a co-regulator, demonstrating its role in inhibiting and modifying the expression of the vitamin D receptor (VDR). The PIAS family was also identified as a novel interacting partner for cleavable isoforms of the receptor tyrosine kinase ErbB4 ICD [15]. Around this time, PIAS1 overexpression was first reported in the nucleus of prostate cancer cells [16]. New research recognized PIAS1 as a key regulator of non-mutational myelo-erythroid gene inactivation in hematopoietic stem cells (HSCs) and lymphoid progenitors [17]. Later, the protein necdin was reported to counteract the proapoptotic activities of PIAS1 [18].
The antiviral efficacy of PIAS4 and PIAS1 as intrinsic antiviral factors against intracellular viral infections was also investigated [19]. In herpes simplex virus 1 infection, PIASy was found to be overexpressed and localized to nuclear domains containing the viral genome. The SIM domain of PIAS4 was shown to be responsible for nuclear localization within the viral genome, while its expression increase in replication compartments depended on the SAP domain or LxxLL motif [20]. Compared to other PIAS family members, PIAS3 was found to be elevated in fibroblast-like synoviocytes (FLSs) and STs derived from patients with rheumatoid arthritis (RA), a chronic inflammatory joint disease [21]. This discovery demonstrated PIAS3, along with PIAS4, as positive regulators of hypoxia-inducible factor (HIF-1α)-mediated transcription [22]. Research also indicated that PIASxα was significantly lower in osteosarcoma, with a focus on its inhibitory mechanism in osteosarcoma through downregulation of key cell cycle regulators like cyclin D kinase in nude mouse tumor models [23].
A novel molecular mechanism for PIAS was reported for the first time in kuruma shrimp (Marsupenaeus japonicus), identifying it as a negative regulator of transcription factors by inhibiting STAT phosphorylation and nuclear translocation [2]. Differential expression of PIAS family members was observed in breast cancer, with PIAS2 and PIAS3 being downregulated, while PIAS4 showed a contradictory trend [24 (Figure 1).
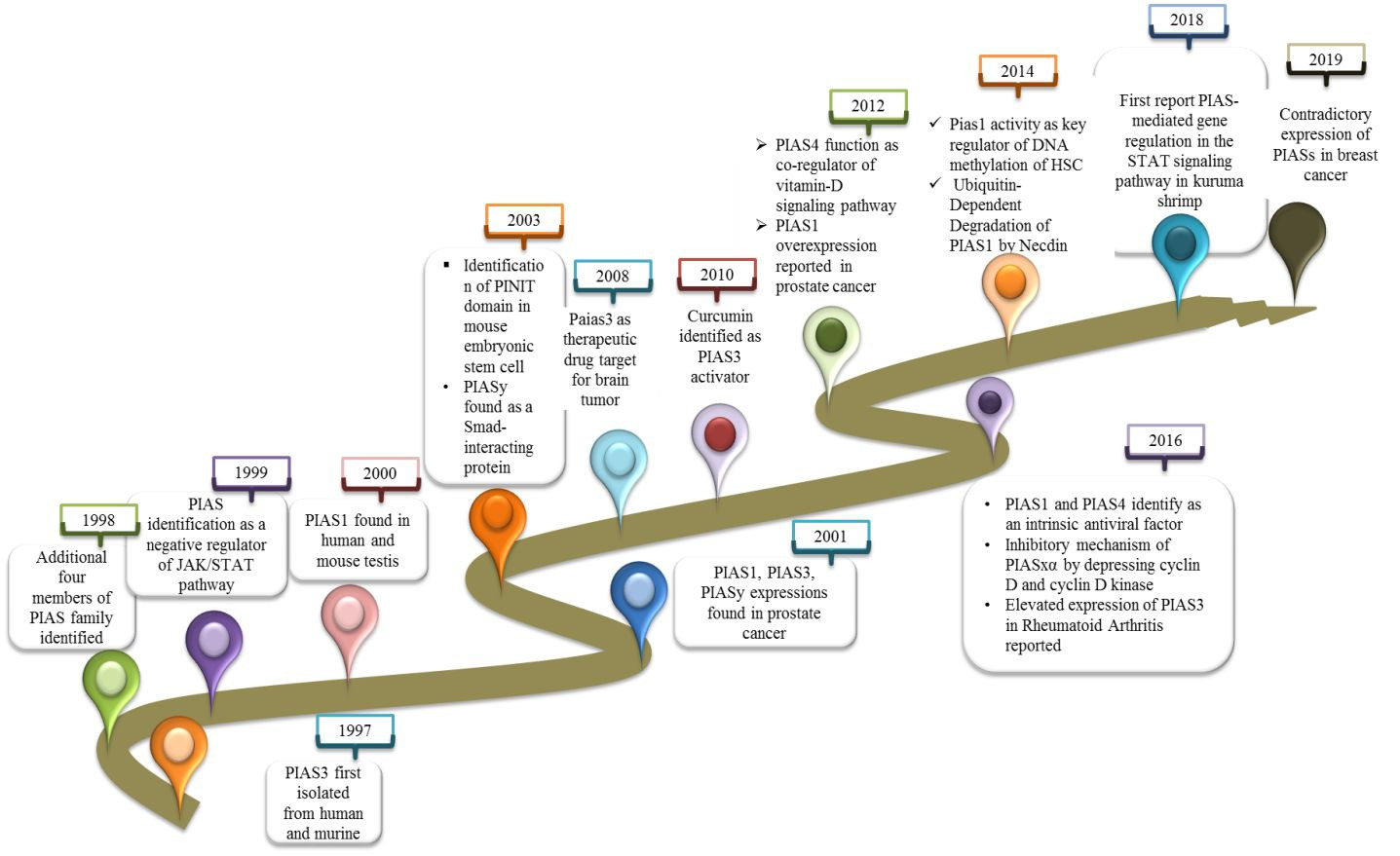 Timeline of PIAS Protein Discoveries
Timeline of PIAS Protein Discoveries
Figure 1 A timeline illustrating key discoveries related to PIAS proteins and their functions over time, highlighting their evolving understanding in cellular regulation and disease.
3. Structure of the PIAS Family
The PIAS family is characterized by four highly homologous members sharing analogous domains, although the number of amino acids varies among them. PIAS1, with 651 amino acids, has the fewest residues. Genetic variations within exons of PIAS 2, 3, and 4 result in splice variants such as PIAS2ab, PIAS3L, and PIAS4E6, respectively (Table 1). Generally, five distinct domains have been identified within this protein family.
At the N-terminal, PIAS proteins are defined by the scaffold attachment factor (SAF)-A/B domain. The SAP domain, containing 35 amino acids along with the LXXLL signature motif, plays a crucial role in interacting with A/T-rich DNA structures, facilitating protein-DNA interactions [25]. The SAP domain is essential for PIASy’s targeting of lymphoid enhancer factor 1 (LEF1), suggesting its involvement in interactions with additional SUMOylated substrates [11].
The PINIT motif domain is present in most PIAS proteins, and is critical for protein localization [1]. Notably, PIAS4 and its splice variant PIAS4E6 completely lack the PINIT motif. A central domain rich in cysteine residues, known as the zinc-binding domain, is also present. Variations in amino acid numbers within PIAS protein domains are observed; for example, the PIAS3L isoform contains an additional 35 amino acids between the SAP and PINIT domains compared to PIAS3. PIAS2a and PIAS2b differ in the length of their serine/threonine (S/T) rich region (Figure 2) [1].
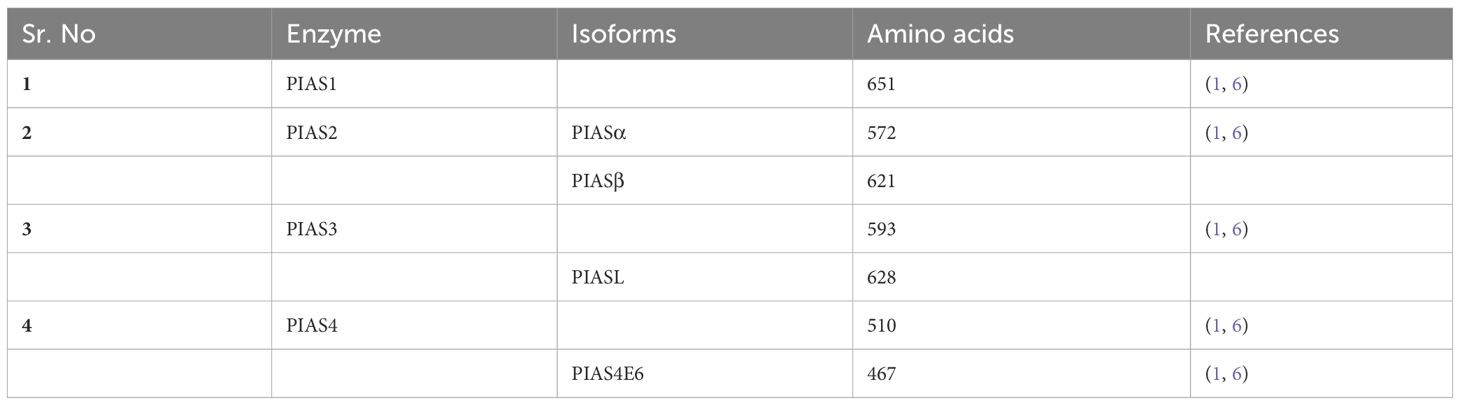 Table of PIAS Family Member Differences
Table of PIAS Family Member Differences
Table 1 Key structural and functional differences between the members of the PIAS family, highlighting variations in domain presence and protein length.
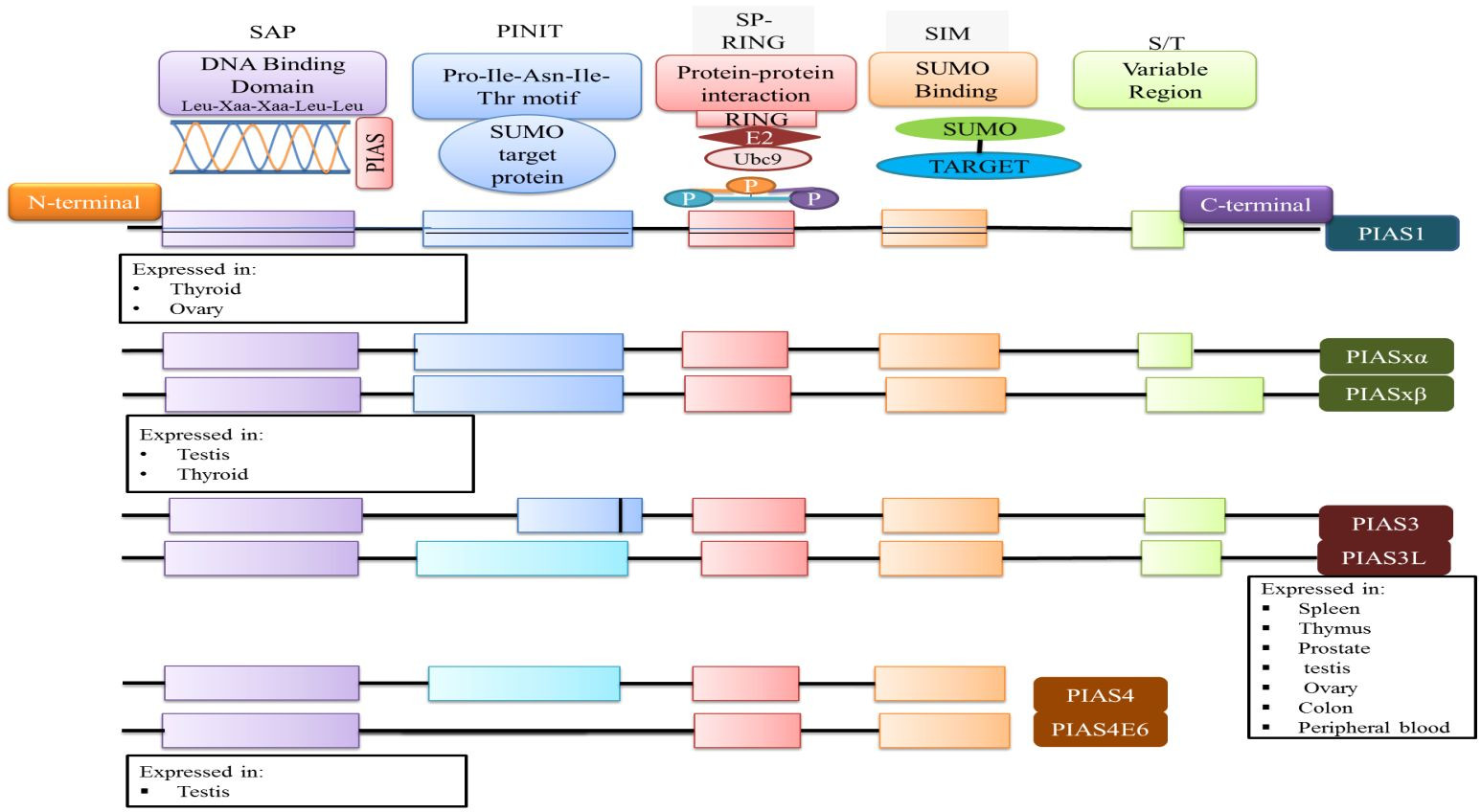 Domain Structure of PIAS Proteins
Domain Structure of PIAS Proteins
Figure 2 Illustrative representation of the domain structure of PIAS proteins, depicting the conserved domains and their functional significance within the PIAS family. Each PIAS member exhibits a unique tissue expression pattern.
4. PIAS Proteins as Transcriptional Regulators
PIAS proteins were initially recognized for their role in inhibiting STAT proteins in 1997 [26]. Cytokine binding to cell surface receptors activates Janus kinases (JAKs), initiating signal transduction pathways including STAT pathways and stress-activated/mitogen-activated protein kinase pathways, which are responsible for diverse cellular responses [2]. In mammals, the STAT family comprises seven members (STAT1-7), each containing conserved tyrosine residues that are phosphorylated by JAKs. The term JAK is derived from “Just Another Kinase,” reflecting its dual domain structure: a catalytic domain and a kinase-like domain. Upon cytokine binding, receptor activation of JAKs leads to trans-phosphorylation of cytoplasmic transcription factors [27]. This phosphorylation induces dimerization of specific STAT proteins, creating docking sites that facilitate their translocation to the nucleus. In the nucleus, STAT dimers either stimulate or suppress regulatory elements to modulate gene transcription.
Biochemical assays have demonstrated that PIAS proteins can block the DNA-binding ability of STATs. PIAS1 interacts with dimeric forms of STAT1, while PIAS2x inhibits the transcriptional potential of both STAT1 and STAT4. Notably, PIAS3 negatively regulates STAT3’s DNA-binding capacity in both homodimeric and heterodimeric forms, thus diminishing its transcriptional activity [27].
The function of PIAS proteins as transcriptional regulators extends beyond the JAK/STAT pathway to include other significant signaling pathways such as NF-κB, p73, p53, and Smad pathways. PIAS proteins modulate the activity of these pathways and subsequently influence downstream gene expression 37088348, 31758961, and 34054823. In the NF-κB pathway, PIAS proteins act as crucial negative regulators. They interact with the p65 subunit of NF-κB in the nucleus, preventing its DNA-binding activity both in vitro and in vivo [28, and PIAS1 can directly bind to DNA to halt NF-κB-mediated transcription. Inflammatory stimuli such as TNF and LPS activate IKKa kinase, which then translocates to the nucleus. Within the nucleus, IKKa interacts with PIAS1, leading to PIAS1 phosphorylation at Ser90. The SUMO E3 ligase activity of PIAS1 is essential for IKKa-mediated PIAS1 phosphorylation. Following phosphorylation, PIAS1 dissociates from IKKa and binds to the promoters of PIAS1-regulated genes, contributing to transcriptional repression [29.
PIAS proteins also integrate signals from other signaling pathways to indirectly influence NF-κB activity. Furthermore, these proteins interact with p73, a member of the p53 tumor suppressor family, playing a significant role in modulating p73 activity through SUMOylation. This modification alters p73’s transcriptional regulation and protein-protein interactions, impacting cellular processes like apoptosis and differentiation [5, 30]. PIAS SUMOylates p73α, reducing its transcriptional activity on genes such as Bax and MDM in HEK293 cells during the G1-to-S phase transition of the cell cycle [31]. Thus, PIAS significantly represses the transcriptional activity of p53, promoting apoptosis by upregulating Bax levels [32 (Figure 3).
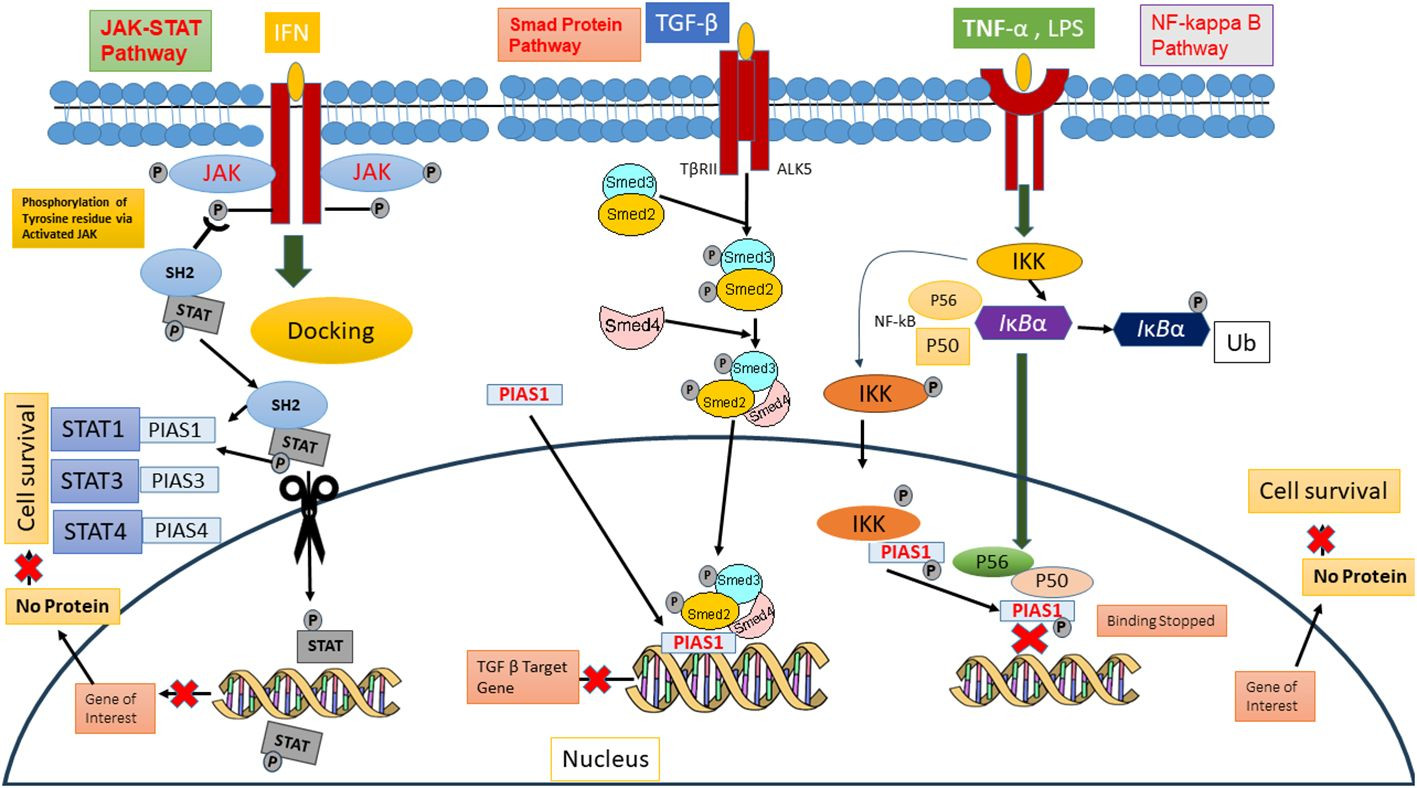 PIAS as Downregulators of Signaling Pathways
PIAS as Downregulators of Signaling Pathways
Figure 3 A schematic representation of PIAS proteins acting as downregulators of key cellular signaling pathways, including JAK/STAT, Smad, and NF-κB pathways, illustrating their broad regulatory roles.
5. The Process of SUMOylation
SUMOylation and ubiquitination are critical components of the ubiquitin-proteasome system, playing significant roles in protein homeostasis and signal transduction [33]. Small Ubiquitin-like Modifier (SUMO) is involved in a crucial covalent modification process where SUMO is attached to a target protein at a lysine residue through an enzymatic cascade [34]. SUMO modification typically regulates protein-protein interactions by facilitating the recruitment of binding partners that contain specific SUMO-interaction motifs (SIMs) [35]. Mammals express four SUMO isoforms: SUMO 1-4 [36. SUMOylation regulates a wide array of biological processes, including protein structure stabilization, DNA damage repair, carcinogenesis, embryonic development, cell proliferation, immune responses, and apoptosis [37.
The enzymatic cascade of SUMOylation involves E1 (activator), E2 (conjugase), and E3 (ligase) enzymes [35]. In humans, the SUMOylation process begins with the proteolytic cleavage at the carboxyl-terminal by sentrin-specific protease 1 (SENP), exposing a terminal diglycine GG motif. The SUMO activation enzymes E1 and E2 form an ATP-dependent heterodimer composed of Aos1-Uba2 and SAE1-SAE2 proteins, respectively. To activate SUMO, Aos1/Uba2-mediated adenylation, driven by ATP, stimulates the linkage of its carboxyl group with SAE2/Uba2 heterodimers. E1 and E2 catalytic cysteine residues converge via the E2 enzyme Ubc9 to promote thioester transfer. However, the precise mechanism remains to be fully elucidated [38]. SUMO E3 ligase plays a pivotal role in substrate targeting; it binds to specific lysine residues in the substrate, and this conjugation can lead to poly-SUMOylation [39. The reversibility of SUMOylation is indicated by the removal of the SUMOglycine residue from the lysine substrate by the SUMO family of proteases [36.
5.1. SUMOylation and Cancer
SUMOylation, a post-translational modification, has emerged as a critical mechanism in regulating numerous biological processes, especially in vertebrates [40]. Dysregulation of SUMOylation is implicated in various age-related disorders and cancer development [41. The SUMO machinery is often overexpressed in cancer cells, contributing to tumorigenesis. E1-conjugated enzymes have been found to be overexpressed in colorectal cancer, and mechanistic studies have shown that the catalytic subunit of E1 (SAE2) can reduce tumor initiation [42].
Recent evidence indicates that SUMOylation of Grb2 (growth factor receptor-bound protein 2) is crucial for the amplification of the Ras/MEK/ERK cascade. Grb2 SUMOylation recruits Sos1 (son of sevenless homolog 1), forming a Grb2-Sos1 complex that initiates the RAS/MEK/MAPK signaling pathway. This pathway plays a pivotal role in carcinogenesis, cell migration, and tumor development [43].
Mutations in regulatory genes like MYC are involved in cell proliferation and tumorigenesis. Myc stimulates SAE1 transcription, and research has demonstrated a synthetic lethality between Myc activation and silencing of SAE1/2 enzymatic activity. In cancer cells, downregulation of SAE1/2 coupled with elevated Myc levels significantly reduces metastasis [36. Furthermore, overexpression of SAE1/2 and Ubc9 has been observed in hepatocellular carcinoma and pancreatic and breast cancers [44. While the involvement of SUMOylation in various cancers is well-documented, the following sections will focus on the specific interaction between PIAS and SUMO in cancer.
5.2. PIAS/SUMO Interaction in Cancer
The most frequently reported PIAS family members in cancer development are PIAS1 and PIAS3. These proteins function as SUMO-specific ligases and trigger the SUMOylation of both tumor suppressor p53 and proto-oncogenes in non-small cell lung carcinoma. PIASy inhibits p53-mediated transactivation without affecting its apoptosis-inducing ability [45]. In contrast, the SUMO E3 ligase PIAS3-Smurf2 SUMOylation pathway suppresses the invasiveness of breast cancer cell-derived organoids. Reduced PIAS3 expression in breast tumors promotes the PIAS3-Smurf2 pathway in tumor progression and metastasis; however, the precise PIAS3-Smurf2 SUMOylation mechanism in breast tumor metastasis requires further investigation [46].
PIASxα is identified as a novel ligase that reduces ubiquitination of PTEN, thereby increasing PTEN protein stability. PTEN regulates the cell cycle by inhibiting the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway [47. Additionally, overexpression of PIAS3xα dysregulates cyclin D kinase (CDK), inhibiting cell proliferation [23. PIAS3xα deficiency may be linked to PTEN ubiquitination and CDK/cyclin D overexpression, potentially promoting uncontrolled cell division. PIAS1, a putative SUMO E3 ligase, extends the half-life of breast cancer amplified sequence 1 (AIB1) and is excessively expressed in approximately 60% of breast tumors. In breast tumors, AIB1 modulates estrogen receptor α (ERα)-mediated gene expression. AIB1 SUMOylation, catalyzed by PIAS1, inhibits AIB1 activity by reducing its interaction with ERα. The therapeutic potential of PIAS1-mediated AIB1 SUMOylation in breast cancer warrants further investigation [48].
5.3. PIAS1 SUMOylation: Cancer Development and Progression
PIAS proteins have diverse functions including cell proliferation, differentiation, apoptosis, tumor development, and immune responses [2]. Cancer proliferation, progression, and therapeutic responses are influenced by the interaction between malignant cells and the tumor microenvironment. Proteins crucial in tumorigenesis often rely on SUMOylation [49]. PIAS1 and PIASxα co-localize with resident proteins of PML nuclear bodies, which are known to be enriched in SUMO1 and SUMO2. The Box2-CC domain of PML facilitates its interaction with PIAS1 and PIASxα. Neither PIASxα nor point mutations at the SUMOylation site affect PIAS1-mediated PML degradation [Figure 4]. Casein kinase II (CK2) overexpression and physical interactions with other proteins play an important role in tumorigenesis. Aberrant interaction between PIAS1 and CK2 leads to ubiquitin-proteasomal degradation of the tumor suppressor protein PML [50].
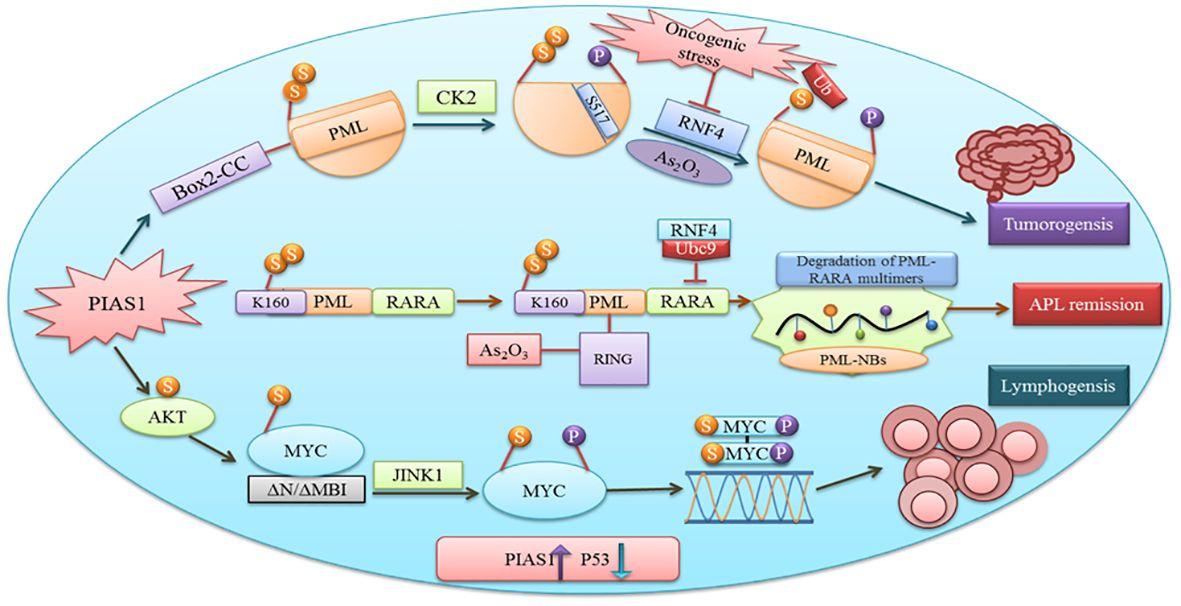 PIAS1 in Tumorigenesis and Lymphomagenesis
PIAS1 in Tumorigenesis and Lymphomagenesis
Figure 4 Diagram illustrating the role of PIAS1 in promoting tumorigenesis through SUMOylation and proteasomal degradation of PML. It also depicts the antagonistic function of PIAS1 in acute promyelocytic leukemia (APL) remission via RNF4-mediated ubiquitin degradation, and the contribution of PIAS1-mediated SUMOylation of MYC to B-cell lymphomagenesis by recruiting JNK1.
SUMOylation also regulates hexokinase 2 at K315 and K492 binding sites. SUMO-defective hexokinase 2 preferentially binds to mitochondria, enhancing glucose consumption and lactate production while downregulating mitochondrial respiration. This metabolic reprogramming supports prostate cancer cell proliferation and protects cells from chemotherapy-induced apoptosis [51]. SUMOylation regulates YTHDF2 binding at the K571 site, promoting mRNA degradation and tumor progression by increasing its binding affinity with m6A-modified mRNA [52]. Monensin, an antibiotic, exhibits anti-ovarian cancer activity by inhibiting the MEK-ERK pathway and enhancing MEK1 SUMOylation [53]. In another study, SYNJ2BP-COX16 promoted breast cancer progression via phosphorylation with DRP1, mitochondrial fission, and SUMOylation at the K107 residue, identifying SYNJ2BP-COX16 as a potential therapeutic target for breast cancer [54]. Similarly, Ginkgolic acid has shown therapeutic potential by inhibiting the growth and invasion of various cancers by hindering IGF-1R SUMOylation [54].
MYC, upon binding to the DNA element CACGTG and dimerization with MAX, acts as a transcriptional activator [55]. Gene translocation or amplification promotes MYC oncogenic activities in numerous human cancers. Research suggests SUMOylation is essential for tolerating aberrant MYC activation [56]. Physical interaction between PIAS1 SUMO E3 ligase and MYC enhances its carcinogenic activity in lymphomas. PIAS1 SUMO E3 ligase promotes MYC transcriptional activity through MYC SUMOylation, upregulates phosphorylated MYC at S62, and provides a docking site for protein kinase JINK1 [57]. Overexpression of PIAS1 and MYC has been observed in stimulated B cells, a substantial subset of primary B-cell lymphomas, and other cancer types. Furthermore, inhibiting SUMOylation induces apoptosis in MYC-dependent lymphoma cells, suggesting it as a promising therapeutic avenue [58].
The antagonistic role of specific PIAS1-mediated SUMOylation stabilizes PML-RARA, which is necessary for its therapeutic action in acute promyelocytic leukemia (APL). Subsequent binding of the RNF4 ubiquitin E3-ligase to poly-SUMOylated PML-RARA results in proteasomal degradation and APL remission [50]. Thus, PIAS1 is crucial for PML-RARA degradation, playing a complex role that can be both pro- and anti-tumorigenic depending on the context.
5.4. PIASY Ligase as Suppressor of Von Hippel–Lindau
PIAS4 also functions as a specific E3-type SUMO ligase. Von Hippel–Lindau (VHL) protein plays a critical role in tumor suppression, which is compromised upon SUMOylation. VHL deficiency leads to constitutive activation of hypoxia-inducible factor (HIF) and subsequent expression of HIF target genes, causing tumor formation. Three PIAS family members are crucial for regulating the hypoxia signaling pathway. Under hypoxic conditions, HIF-1α translocates to the nucleus and binds with PIAS4, which SUMOylates HIF1α within the C-terminal. SUMOylation enhances HIF1α-dependent transcriptional activity, facilitates VHL binding, and leads to uniform degradation under hypoxic conditions [59]. Research has shown that PIAS4 stimulates HIF1α signaling through interaction and SUMOylation of VHL in pancreatic cancer and renal clear-cell carcinoma [60].
Synovial sarcoma, a soft tissue cancer, involves the activation of oncoproteins like SYT-SSX1 and SYT-SSX2 due to chromosomal translocation. NOCOA3, a protein upregulated by SYT-SSX1, contributes to the development of various cancers. The interaction of SUMO E3 ligase PIAS4 with SYT-SSX1 increases the SUMOylation of its substrate NOCOA3 and its binding protein NEMO in synovial sarcoma. PIAS4-mediated SUMOylation is associated with the upregulation of nuclear receptor coactivator 3, which triggers adverse effects in normal cells [61].
5.5. Smad and PIAS Protein Interaction Promote Cancer
SMADs are a class of proteins that transmit extracellular signals from the TGF-β ligand to the nucleus to regulate transcription [62]. PIAS proteins have been reported to modulate the transcriptional function of SMADs, mediating TGF-β biological activities [63.
TGF-β serves as a key regulator in cancer progression and immune evasion. USP8 promotes metastasis, and its therapeutic inhibition can suppress metastatic activity 35811497. PRMT5 interacts with SMAD4, and SMAD4 R361 methylation plays a role in controlling TGF-β signaling during metastasis [64].
In various cancer cells, PIAS1 enhances the transcriptional function of the Smad2/Smad4 protein complex. The TGF-β-activating R-Smad/Co-Smad complex promotes the inhibition of cyclin-dependent kinase (CDK), inducing cell growth arrest through direct activation of the promoter region of the p21WAF1/Cip1 gene. PIAS1 is crucial for zinc-induced Smad4 pathway activation. Impairment in the Smad pathway contributes to carcinogenesis due to the escape from growth inhibition [63; however, the precise mechanism of PIAS1 interaction with Smad still needs further investigation.
PIAS3 is downregulated by Smad6 via the ubiquitin-proteasome pathway. PIAS3 interacts with Smad2, 3, 4, and 6 members of the SMAD family. In the case of Smad6, the MH2 domain and the PIAS3 RING domain are responsible for their interaction, which degrades PIAS3 and promotes STAT3 activity in glioblastoma. Another protein family member, Smurf E3 ubiquitin-protein ligase catalytic domain, interacts with the PY motif of Smad6 and facilitates PIAS3 degradation, promoting tumor growth, invasion, and survival [65].
5.6. PIAS3 as a Target of MicroRNAs in Tumors
MicroRNAs (miRNAs) are single-stranded RNAs that function as post-transcriptional modulators of gene expression. MiR-18a binds to the potential binding site in the 3′UTR of PIAS3. During gastric adenocarcinoma development, upregulation of miR-18a and miR-21 suppresses PIAS3 expression. Recent work has shown that overexpression of miR-199a-5 negatively targets PIAS3 and p27 in osteosarcoma. Upregulation of miR-199a-5 enhances STAT3 phosphorylation, and ectopic overexpression of p27 delays the G1-S phase transition in the cell cycle. Activated STAT3 helps tumor cells evade apoptosis and supports their proliferation [66]. Therefore, miRNA inhibitors could be a potential therapeutic approach in cancer therapy [Figure 5].
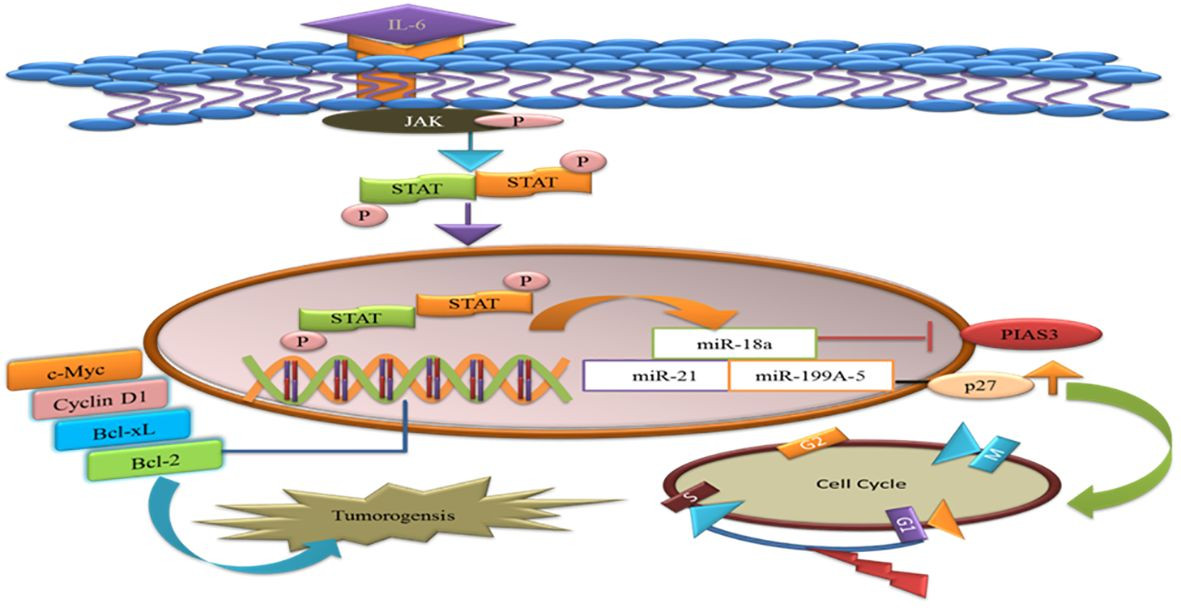 MicroRNA Targeting of PIAS3 in Tumorigenesis
MicroRNA Targeting of PIAS3 in Tumorigenesis
Figure 5 A visual summary of how microRNAs target PIAS3, leading to the upregulation of STAT3, which in turn promotes anti-apoptotic genes and tumorigenesis. The diagram also illustrates miR-a99a-5 targeting p27 and its effect on delaying the G1/S phase of the cell cycle.
Tumor exosome-derived miR-9 and miR-181a activate the JAK/STAT pathway by targeting PIAS3 and SOCS3, thus promoting the expression of eMDSCs and potentially serving as a novel target for IL-6 high breast cancer treatment [67]. MiR-199a-5p and microRNA-543 participate in cancer progression and proliferation by targeting PIAS3 in cervical cancer and colorectal cancer [68, 69].
6. Modulated Expression of PIAS Proteins in Different Cancers
Recent research has highlighted the correlation between aberrant expression of PIAS proteins and clinicopathological conditions in various cancers.
6.1. PIAS1
Antagonistic expression of PIAS1 is implicated in aberrant signaling pathways during carcinogenesis. Since PIAS proteins contain an SP-RING domain exhibiting SUMO E3 ligase activity, PIAS1-dependent SUMOylation is observed in the modulation of numerous oncogenes and tumor suppressor genes like AKT, BRCA1, and BRCA2 [57]. PIAS1 acts as a cell cycle regulator, catalyzing the SUMOylation of tumor suppressors p53 and p73, thereby promoting cell proliferation [70]. In lung cancer and osteosarcoma cells, PIAS1, primarily overexpressed during the S phase, may attach to and SUMOylate p73, hindering its transcriptional activity, followed by a reduction in p21 [16]. Overexpression of PIAS1 has been reported in lung cancer [71. The PIAS1 gene contributes to the nuclear accumulation of focal adhesion kinase FAK, where FAK accelerates p53 knockdown, ultimately promoting non-small cell lung cancer (NSCLC) progression [72]. PIAS1 is also overexpressed in endometrial cancer (EC), and the upregulation of miR-182-5p and miR-96-5p downregulates PIAS1 levels in EC [73].
6.2. PIAS2
PIAS2 gene expression is significantly reduced in cancerous tissues compared to adjacent non-cancerous tissues [24]. Low levels of PIASxα, a splice variant of PIAS2, are associated with tumorigenesis and cell proliferation in osteosarcoma tissues [23]. PIAS2 interacts with UXT protein, a crucial co-regulator of transcription factors such as the androgen receptor (AR), in both the cytoplasm and nucleus of human cervical carcinoma cells [74]. PIAS2 also interacts with ZFHX3 and enhances its activity in cell proliferation in cancer cells [75].
6.3. PIAS3
PIAS3 is generally expressed in numerous human tissues, including the spleen, thymus, prostate, testis, ovary, colon, and peripheral blood. The PINIT domain found in PIAS3 can induce apoptosis by inhibiting the transcriptional activity of STAT3 [2]. Multiple lines of evidence suggest that low PIAS3 expression supports cancer cell proliferation or promotes tumorigenesis. PIAS3 mRNA expression is often silenced, influencing the JAK/STAT signaling cascade in gastric, medulloblastoma, breast, and lung cancers [76]. PIAS3 levels are negatively correlated with aberrant expression of STAT3 and its downstream targets, such as survivin, Bcl-xL, and c-Myc, in colorectal cancer [77]. In lymphocytic leukemia, aberrant signaling pathways in the tumor microenvironment, including ZAP-70 protein, MAPK, and STAT3, stimulate the expression of post-translational regulator microRNA (miR-21) and other tumor suppressor genes (PTEN, PDCD4, and PIAS3). Upregulation of miR-21 expression with interleukin-4 (IL-4) promotes oncogenic processes through downregulation of tumor suppressor genes PTEN, PDCD4, and PIAS3 [78]. Another microRNA, miR-18a, is negatively linked with PIAS3 expression in malignant mesothelioma [79]. In glioblastoma, tri-partite motif-containing protein 8 (TRIM8) and nuclear-Smad6 mediate ubiquitination, leading to PIAS3 degradation, which in turn promotes STAT3 activation [80].
6.4. PIAS4
PIAS4 is widely expressed in the testis and is the smallest protein among PIASs, encoding one splice variant, PIAS4E6 [81]. Upregulation of PIAS4 has been reported in human cancers. PIAS4 interacts with and inhibits p53-mediated transactivation of its downregulators like Bax and p21 in NSCLC, inhibiting apoptosis [60]. PIAS4, as a transcriptional co-repressor of the androgen receptor, interacts with the DNA-binding domain of AR, essential for prostate cancer development and progression [Figure 6].
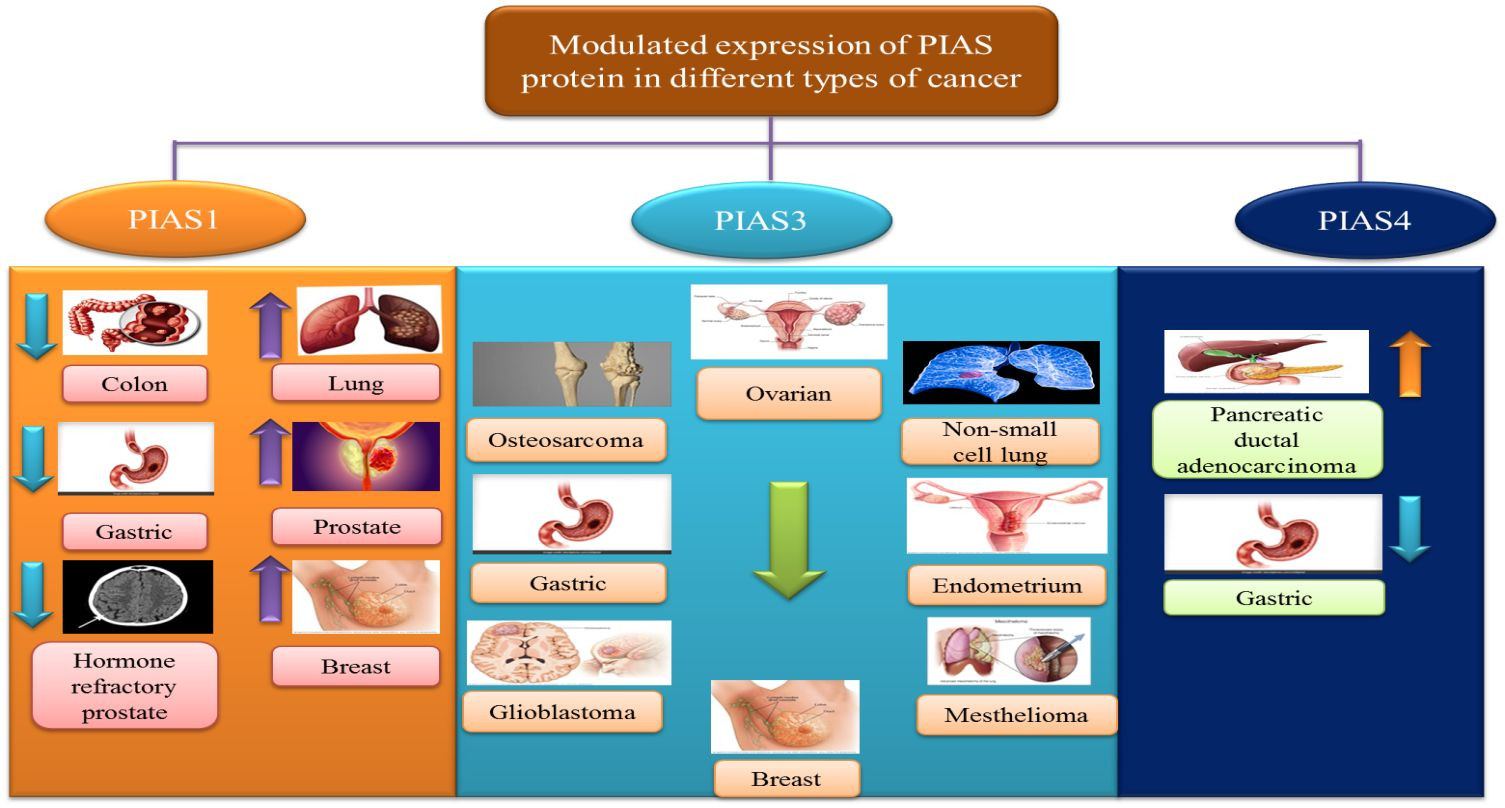 Modulated PIAS Protein Expression in Cancer
Modulated PIAS Protein Expression in Cancer
Figure 6 A summary diagram illustrating how controversial and modulated expressions of different PIAS proteins contribute to cancer development across various cancer types.
7. PIAS1 Suppresses Invasive Tumor Growth via SnoN SUMOylation
PIAS proteins can inhibit caspase activity, neutralize pro-caspase activation, and function as ubiquitin ligases. PIAS proteins are involved in the negative regulation of apoptosis through proteasomal degradation of pro-apoptotic proteins and STATs, as reported in several cancers [82]. PIAS1 and TIF1gamma promote SnoN SUMOylation and suppress epithelial-mesenchymal transition (EMT) in cancer cells, although the precise regulation of EMT by PIAS1 is still being elucidated [83].
The prognostic value of PIAS1 has revealed detailed mechanisms of this protein in SnoN SUMOylation, suggesting significant opportunities for breast cancer therapy [84]. SnoN is a negative feedback inhibitor of transforming growth factor beta (TGF-β) signaling. TGF-β’s biphasic role in advanced cancer phases allows cells to metastasize by inducing EMT [85]. During EMT, cancer cells migrate from the primary tumor site and invade distant sites. TGF-β requires Smad- and MAD-related protein family members, Smad2 and Smad3, to transduce signals to the nucleus. Thus, the TGF-β-induced EMT pathway stimulates tumor progression in later stages of epithelial tissues. SnoN and SUMO E3 ligase PIAS1 have emerged as EMT regulators [86]. In epithelial cells, PIAS1-SnoN SUMOylation inhibits TGF-β-induced EMT, suggesting a fundamental role for SnoN-SUMOylation in cancer progression. Previous research has identified PIAS1 as a biomarker for breast cancer patients [85]. The inhibition of TGF-β-induced EMT via SUMO E3 ligase PIAS1 and SnoN SUMOylation raises the question of whether low levels and specific subcellular localization of PIAS1 enhance TGF-β-induced EMT and cancer metastasis [Figure 7].
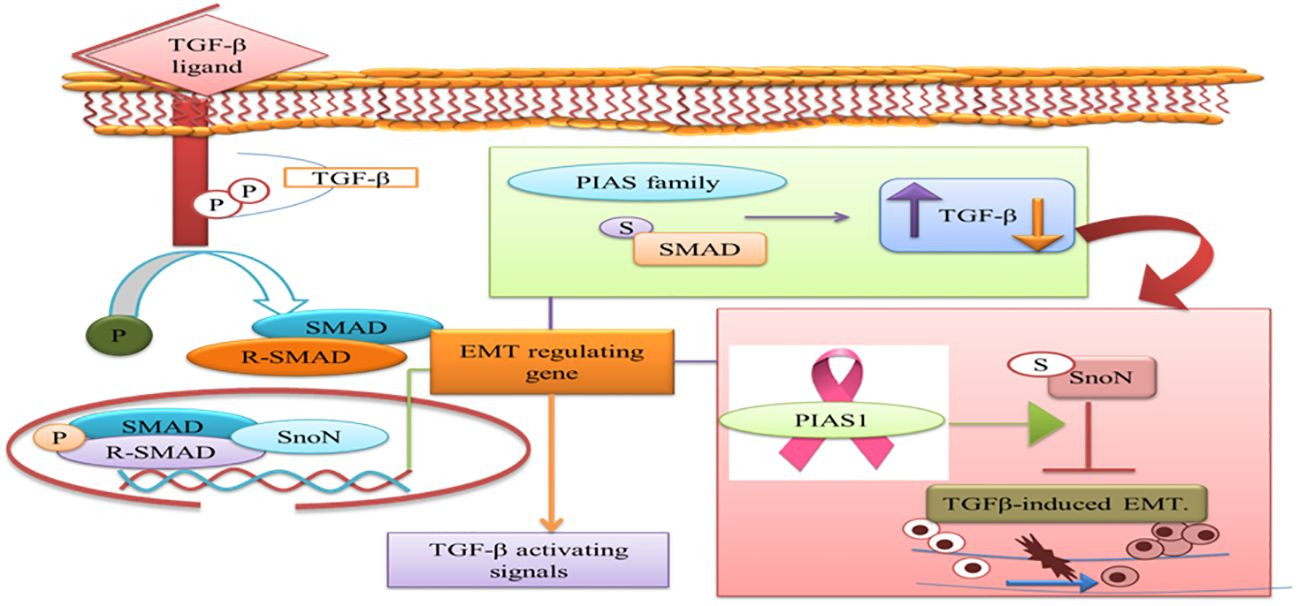 PIAS Family and TGF-β Signaling in EMT
PIAS Family and TGF-β Signaling in EMT
Figure 7 A depiction of the PIAS family’s dual role in TGF-β signaling pathways, which require SMAD proteins for nuclear translocation. The figure highlights PIAS1’s modulation of the TGF-β-induced EMT complex through SnoN SUMOylation. (TGF-β: transforming growth factor beta; PIAS: protein inhibitor of activated STAT; EMT: epithelial-mesenchymal transition).
8. Natural Activators
Downregulation of PIAS3 leads to STAT3 upregulation, promoting multiple oncogenic pathways, making it an important target for cancer therapies [87]. Given these facts, efforts have been made to identify natural activators that can restore PIAS3 levels in cancer cells [88]. Ascochlorin, an isoprenoid antibiotic from the phytopathogenic fungus Ascochyta viciae, induces inhibition of STAT3 and significantly enhances PIAS3 protein expression, substantially suppressing cancer growth in HepG2 cells. Curcumin, another natural compound, enhances PIAS3 expression and inhibits STAT3 phosphorylation in ovarian and endometrial cancer cells [89]. Brassinin (BSN), a phytoalexin first isolated from cabbage, exhibits anti-tumor effects by enhancing PIAS3 expression. BSN inhibits IL-6-induced STAT3 phosphorylation, involving two STAT3 inhibitors: PIAS3 and SOCS-3 [Figure 8]. Paclitaxel, a semi-synthetic taxane from the bark of the Pacific yew tree, is used therapeutically in NSCLC, but its severe side effects necessitate exploring combinations with BSN to reduce chemotherapy lethality in lung cancer treatment [90]. Proteasomal inhibitors bortezomib or marizomib induce caspase 9 and increase PIAS3 expression, initiating apoptosis and inhibiting STAT3 activity in glioblastoma cells [91].
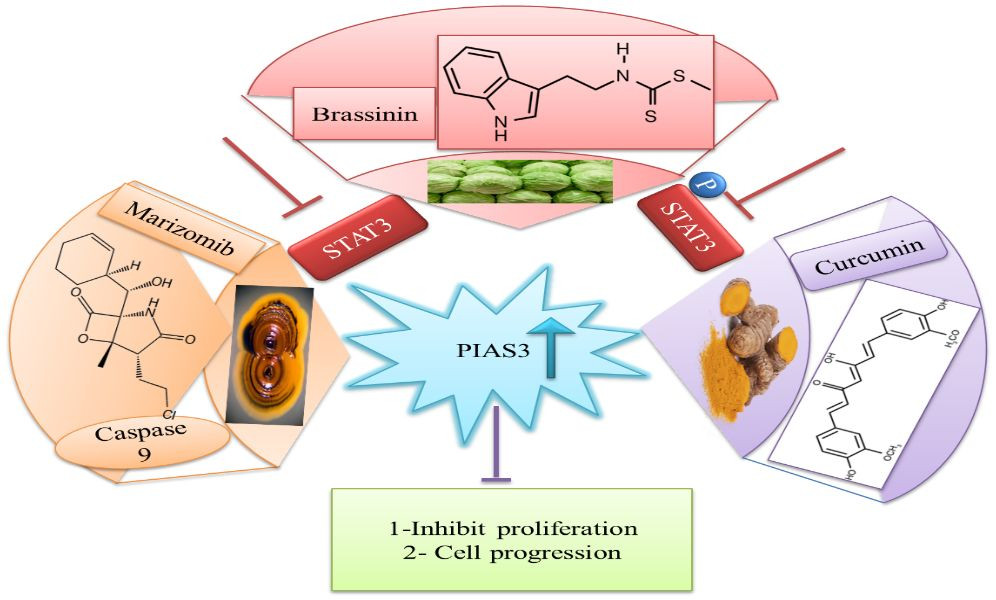 Natural Activators of PIAS3 and STAT3 Regulation
Natural Activators of PIAS3 and STAT3 Regulation
Figure 8 A diagram showing natural activators of PIAS3, such as Ascochlorin, Curcumin, and Brassinin, and their mechanisms in negatively regulating STAT3 expression, offering potential therapeutic strategies. (STAT3: signal transducer and activator of transcription 3; PIAS3: protein inhibitor of activated STAT3).
9. Clinical Applications of PIAS in Cancer
PIAS proteins are differentially expressed in various cancers, making them potential targets for novel cancer treatments. Studies examining PIAS3 mRNA in breast cancer patients and non-cancerous breast tissues showed significantly lower PIAS3 mRNA expression in cancer patients, suggesting PIAS3 mRNA as a crucial factor in breast cancer development [92].
PIAS proteins function as suppressors within the STAT3 signaling pathway, a critical regulator of cellular processes like proliferation, differentiation, and apoptosis. By regulating the STAT3 pathway, PIAS proteins emerge as potential therapeutic targets for STAT3-dependent cancers. Manipulating the STAT3 pathway using PIAS holds promise for treating cancers driven by aberrant STAT3 activity [58].
PIAS1 expression induced by the Ad5/F35 virus in gastric cancer patients reduced cell proliferation and invasion. These results indicate PIAS1 may act as a cancer suppressor to control metastasis [93]. Expression analysis of PIASxα in tissue samples from osteosarcoma patients using RT-qPCR and Western blot revealed reduced PIASxα expression. Overexpression of PIASxα significantly increased apoptosis and decreased tumor formation in mouse models [94].
Inhibitors of miR-182-5p and miR-96-5p can increase PIAS1 expression in endometrial cancer (EC) cells. This PIAS1 level increase plays a key role in inhibiting STAT3 activity. Furthermore, treatment with ectopic PIAS1 expression and STAT3 inhibitors further suppresses STAT3 activity and reduces miR-182-5p and miR-96-5p levels in EC cells. These findings suggest that these inhibitors disrupt the negative feedback loop between PIAS1 and STAT3, offering a promising approach for EC management by targeting this molecular pathway [73]. Quantitative SUMO proteomics analysis following CRISPR/Cas9 knockout of individual PIAS genes provides novel insights into the regulatory roles of PIAS SUMO E3 ligases, elucidating both specific and overlapping mechanisms governing cell proliferation and the cell cycle [95].
Curcumin, a dihydroxyphenolic compound with anti-cancer activity, increases PIAS3 expression in normal ovarian and endometrial cells, while tumor cells show considerably decreased expression. Curcumin enhances PIAS3 expression in tumor cells, thus inhibiting JAK-STAT signaling through PIAS3 activation, reducing STAT3 phosphorylation and cancer cell growth [89].
Brassinin, a phytoalexin with documented anticarcinogenic, chemopreventive, and antiproliferative potential [96–99], prevents STAT3 signaling by modulating PIAS3, leading to reduced cancer growth (b). Targeting PIAS proteins with specific inhibitors in the future could be a promising therapeutic strategy to reduce cell proliferation and treat cancer [90].
10. Conclusion and Future Challenges
Recent evidence underscores the antagonistic role of PIAS family members as SUMO E3 ligases, positioning them as key regulators within complex oncogenic networks. Emerging reports indicate that PIAS protein expression varies across different cancer types. Therefore, targeting PIAS proteins and their downstream targets is a promising novel treatment strategy for cancer therapy. Current research focuses on developing pharmacological and natural inhibitors and activators to modulate PIAS expression. However, more research should concentrate on elucidating the molecular mechanisms of natural PIAS3 activators. The interactions of PIAS1 and PIAS2 with SMAD, and PIAS1, PIAS2, and PIAS3 with Von Hippel–Lindau, warrant further investigation. Detailed studies on the possible mechanisms of miRNA and PIAS3 interactions are also needed. A more comprehensive understanding of the overall expression patterns of PIAS2 and PIAS4 in tumors is crucial. Future studies screening drugs to stabilize antagonistic PIAS protein expression could offer potential therapeutic indices for cancer treatment.
Author Contributions
XL: Writing – review & editing, Funding acquisition, Conceptualization. AR: Writing – review & editing, Validation, Supervision. FS: Writing – original draft. MH: Writing – review & editing, Writing – original draft, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant Number 31870758) and the Jilin Provincial Science and Technology Department (Grant Number 20200201025JC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1 Rytinki MM, Kaikkonen S, Pehkonen P, Jaaskelainen T, Palvimo JJ. PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci. (2009) 66:3029–41. doi: 10.1007/s00018-009-0061-z
CrossRef Full Text | Google Scholar
2 Niu GJ, Xu JD, Yuan WJ, Sun JJ, Yang MC, He ZH, et al. Protein inhibitor of activated STAT (PIAS) negatively regulates the JAK/STAT pathway by inhibiting STAT phosphorylation and translocation. Front Immunol. (2018) 9:2392. doi: 10.3389/fimmu.2018.02392
CrossRef Full Text | Google Scholar
3 Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, et al. Inhibition of Stat1-mediated gene activation by PIAS1. Proc Natl Acad Sci U.S.A. (1998) 95:10626–31. doi: 10.1073/pnas.95.18.10626
CrossRef Full Text | Google Scholar
4 Murphy JM, Tannahill GM, Hilton DJ, Greenhalgh CJ. The negative regulation of JAK/STAT signaling. In: Handbook of cell signaling. Academic Press. Elsevier (2010). p. 467–80. doi: 10.1016/B978-0-12-374145-5.00064-4
CrossRef Full Text | Google Scholar
5 Wu M, Song D, Li H, Yang Y, Ma X, Deng S, et al. Negative regulators of STAT3 signaling pathway in cancers. Cancer Manag Res. (2019) 11:4957–69. doi: 10.2147/CMAR
CrossRef Full Text | Google Scholar
6 Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science. (1997) 278:1803–5. doi: 10.1126/science.278.5344.1803
CrossRef Full Text | Google Scholar
7 Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioessays. (1999) 21:47–52. doi: 10.1002/(SICI)1521-1878(199901)21:11.0.CO;2-4
CrossRef Full Text | Google Scholar
8 Tan J, Hall SH, Hamil KG, Grossman G, Petrusz P, Liao J, et al. Protein inhibitor of activated STAT-1 (signal transducer and activator of transcription-1) is a nuclear receptor coregulator expressed in human testis. Mol Endocrinol. (2000) 14:14–26. doi: 10.1210/mend.14.1.0408
CrossRef Full Text | Google Scholar
9 Junicho A, Matsuda T, Yamamoto T, Kishi H, Korkmaz K, Saatcioglu F, et al. Protein inhibitor of activated STAT3 regulates androgen receptor signaling in prostate carcinoma cells. Biochem Biophys Res Commun. (2000) 278:9–13. doi: 10.1006/bbrc.2000.3753
CrossRef Full Text | Google Scholar
10 Gross M, Liu B, Tan J, French FS, Carey M, Shuai K. Distinct effects of PIAS proteins on androgen-mediated gene activation in prostate cancer cells. Oncogene. (2001) 20:3880–7. doi: 10.1038/sj.onc.1204489
CrossRef Full Text | Google Scholar
11 Sachdev S, Bruhn L, Sieber H, Pichler A, Melchior F, Grosschedl R. PIASy, a nuclear matrix-associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev. (2001) 15:3088–103. doi: 10.1101/gad.944801
CrossRef Full Text | Google Scholar
12 Duval D, Duval G, Kedinger C, Poch O, Boeuf H. The ‘PINIT’ motif, of a newly identified conserved domain of the PIAS protein family, is essential for nuclear retention of PIAS3L. FEBS Lett. (2003) 554:111–8. doi: 10.1016/S0014-5793(03)01116-5
CrossRef Full Text | Google Scholar
13 Long J, Wang G, Matsuura I, He D, Liu F. Activation of Smad transcriptional activity by protein inhibitor of activated STAT3 (PIAS3). Proc Natl Acad Sci U.S.A. (2004) 101:99–104. doi: 10.1073/pnas.0307598100
CrossRef Full Text | Google Scholar
14 Ehrmann J, Strakova N, Vrzalikova K, Hezova R, Kolar Z. Expression of STATs and their inhibitors SOCS and PIAS in brain tumors. In vitro and in vivo study. Neoplasma. (2008) 55:482–7.
15 Sundvall M, Korhonen A, Vaparanta K, Anckar J, Halkilahti K, Salah Z, et al. Protein inhibitor of activated STAT3 (PIAS3) protein promotes SUMOylation and nuclear sequestration of the intracellular domain of ErbB4 protein. J Biol Chem. (2012) 287:23216–26. doi: 10.1074/jbc.M111.335927
CrossRef Full Text | Google Scholar
16 Hoefer J, Schafer G, Klocker H, Erb HH, Mills IG, Hengst L, et al. PIAS1 is increased in human prostate cancer and enhances proliferation through inhibition of p21. Am J Pathol. (2012) 180:2097–107. doi: 10.1016/j.ajpath.2012.01.026
CrossRef Full Text | Google Scholar
17 Ghaffari S. PIAS adds methyl-bias to HSC-differentiation. EMBO J. (2014) 33:93–5. doi: 10.1002/embj.v33.2
CrossRef Full Text | Google Scholar
18 Gur I, Fujiwara K, Hasegawa K, Yoshikawa K. Necdin promotes ubiquitin-dependent degradation of PIAS1 SUMO E3 ligase. PloS One. (2014) 9:e99503. doi: 10.1371/journal.pone.0099503
CrossRef Full Text | Google Scholar
19 Brown JR, Conn KL, Wasson P, Charman M, Tong L, Grant K, et al. SUMO ligase protein inhibitor of activated STAT1 (PIAS1) is a constituent promyelocytic leukemia nuclear body protein that contributes to the intrinsic antiviral immune response to herpes simplex virus 1. J Virol. (2016) 90:5939–52. doi: 10.1128/JVI.00426-16
CrossRef Full Text | Google Scholar
20 Conn KL, Wasson P, Mcfarlane S, Tong L, Brown JR, Grant KG, et al. Novel role for protein inhibitor of activated STAT 4 (PIAS4) in the restriction of herpes simplex virus 1 by the cellular intrinsic antiviral immune response. J Virol. (2016) 90:4807–26. doi: 10.1128/JVI.03055-15
CrossRef Full Text | Google Scholar
21 Lao M, Shi M, Zou Y, Huang M, Ye Y, Qiu Q, et al. Protein inhibitor of activated STAT3 regulates migration, invasion, and activation of fibroblast-like synoviocytes in rheumatoid arthritis. J Immunol. (2016) 196:596–606. doi: 10.4049/jimmunol.1403254
CrossRef Full Text | Google Scholar
22 Nakagawa K, Kohara T, Uehata Y, Miyakawa Y, Sato-Ueshima M, Okubo N, et al. PIAS3 enhances the transcriptional activity of HIF-1alpha by increasing its protein stability. Biochem Biophys Res Commun. (2016) 469:470–6. doi: 10.1016/j.bbrc.2015.12.047
CrossRef Full Text | Google Scholar
23 Wang J, Ni J, Yi S, Song D, Ding M. Protein inhibitor of activated STAT xalpha depresses cyclin D and cyclin D kinase, and contributes to the inhibition of osteosarcoma cell progression. Mol Med Rep. (2016) 13:1645–52. doi: 10.3892/mmr.2015.4705
CrossRef Full Text | Google Scholar
24 Taheri M, Oskooei VK, Ghafouri-Fard S. Protein inhibitor of activated STAT genes are differentially expressed in breast tumor tissues. Per Med. (2019) 16:277–85. doi: 10.2217/pme-2018-0070
CrossRef Full Text | Google Scholar
25 Aravind L, Koonin EV. SAP – a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci. (2000) 25:112–4. doi: 10.1016/S0968-0004(99)01537-6
CrossRef Full Text | Google Scholar
26 Zhang S, Li C, Wang W, Wang C, Sun C, Chan S, et al. Functional characterization of a protein inhibitor of activated STAT (PIAS) gene in Litopenaeus vannamei. Fish Shellfish Immunol. (2019) 94:417–26. doi: 10.1016/j.fsi.2019.09.007
CrossRef Full Text | Google Scholar
27 Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. (2017) 15:23. doi: 10.1186/s12964-017-0177-y
CrossRef Full Text | Google Scholar
28 Liu B, Yang R, Wong KA, Getman C, Stein N, Teitell MA, et al. Negative regulation of NF-kappaB signaling by PIAS1. Mol Cell Biol. (2005) 25:1113–23. doi: 10.1128/MCB.25.3.1113-1123.2005
CrossRef Full Text | Google Scholar
29 Liu B, Shuai K. Targeting the PIAS1 SUMO ligase pathway to control inflammation. Trends in pharmacological sciences (2008) 29:505–9. doi: 10.1016/j.tips.2008.07.008
CrossRef Full Text | Google Scholar
30 Ghafouri-Fard S, Hussen BM, Nicknafs F, Nazer N, Sayad A, Taheri M. Expression analysis of protein inhibitor of activated STAT in inflammatory demyelinating polyradiculoneuropathy. Front Immunol. (2021) 12:659038. doi: 10.3389/fimmu.2021.659038
CrossRef Full Text | Google Scholar
31 Omran Z, H.D. M, Abdullah O, Kaleem M, Hosawi S, A.a.-A. F, et al. Targeting post-translational modifications of the p73 protein: A promising therapeutic strategy for tumors. Cancers (Basel). (2021) 13(8):1916. doi: 10.3390/cancers13081916
CrossRef Full Text | Google Scholar
32 Schmidt D, Müller S. Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity. Proc Natl Acad Sci U.S.A. (2002) 99:2872–7. doi: 10.1073/pnas.052559499
CrossRef Full Text | Google Scholar
33 Dai X, Zhang T, Hua D. Ubiquitination and SUMOylation: protein homeostasis control over cancer. Epigenomics. (2022) 14:43–58. doi: 10.2217/epi-2021-0371
CrossRef Full Text | Google Scholar
34 Zhao X. SUMO-mediated regulation of nuclear functions and signaling processes. Mol Cell. (2018) 71:409–18. doi: 10.1016/j.molcel.2018.07.027
CrossRef Full Text | Google Scholar
35 Nayak A, Muller S. SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol. (2014) 15:422. doi: 10.1186/s13059-014-0422-2
CrossRef Full Text | Google Scholar
36 Han ZJ, Feng YH, Gu BH, Li YM, Chen H. The post-translational modification, SUMOylation, and cancer (Review). Int J Oncol. (2018) 52:1081–94. doi: 10.3892/ijo
CrossRef Full Text | Google Scholar
37 Bettermann K, Benesch M, Weis S, Haybaeck J. SUMOylation in carcinogenesis. Cancer Lett. (2012) 316:113–25. doi: 10.1016/j.canlet.2011.10.036
CrossRef Full Text | Google Scholar
38 Pichler A, Fatouros C, Lee H, Eisenhardt N. SUMO conjugation – a mechanistic view. Biomol Concepts. (2017) 8:13–36. doi: 10.1515/bmc-2016-0030
CrossRef Full Text | Google Scholar
39 Sriramachandran AM, Dohmen RJ. SUMO-targeted ubiquitin ligases. Biochim Biophys Acta. (2014) 1843:75–85. doi: 10.1016/j.bbamcr.2013.08.022
CrossRef Full Text | Google Scholar
40 Lara-Ureña N, Jafari V, García-Domínguez M. Cancer-associated dysregulation of sumo regulators: proteases and ligases. Int J Mol Sci. (2022) 23(14):8012. doi: 10.3390/ijms23148012
CrossRef Full Text | Google Scholar
41 Sun Q, Qing W, Qi R, Zou M, Gong L, Liu Y, et al. Inhibition of sumoylation alleviates oxidative stress-induced retinal pigment epithelial cell senescence and represses proinflammatory gene expression. Curr Mol Med. (2018) 18:575–83. doi: 10.2174/1566524019666190107154250
CrossRef Full Text | Google Scholar
42 Du L, Li YJ, Fakih M, Wiatrek RL, Duldulao M, Chen Z, et al. Role of SUMO activating enzyme in cancer stem cell maintenance and self-renewal. Nat Commun. (2016) 7:12326. doi: 10.1038/ncomms12326
CrossRef Full Text | Google Scholar
43 Qu Y, Chen Q, Lai X, Zhu C, Chen C, Zhao X, et al. SUMOylation of Grb2 enhances the ERK activity by increasing its binding with Sos1. Mol Cancer. (2014) 13:95. doi: 10.1186/1476-4598-13-95
CrossRef Full Text | Google Scholar
44 van Doorn BA, van der Does E, Lubsen J, Rijsterborgh H. [Reliability of blood pressure measurements; comparison of an electronic meter and a mercury manometer in family practice]. Ned Tijdschr Geneeskd. (1990) 134:1646–50.
45 Nelson V, Davis GE, Maxwell SA. A putative protein inhibitor of activated STAT (PIASy) interacts with p53 and inhibits p53-mediated transactivation but not apoptosis. Apoptosis. (2001) 6:221–34. doi: 10.1023/A:1011392811628
CrossRef Full Text | Google Scholar
46 Chandhoke AS, Chanda A, Karve K, Deng L, Bonni S. The PIAS3-Smurf2 sumoylation pathway suppresses breast cancer organoid invasiveness. Oncotarget. (2017) 8:21001–14. doi: 10.18632/oncotarget.v8i13
CrossRef Full Text | Google Scholar
47 Wang W, Chen Y, Wang S, Hu N, Cao Z, Wang W, et al. PIASxalpha ligase enhances SUMO1 modification of PTEN protein as a SUMO E3 ligase. J Biol Chem. (2014) 289:3217–30. doi: 10.1074/jbc.M113.508515
CrossRef Full Text | Google Scholar
48 De Silva DD, Rapior S, Hyde KD, Bahkali AH. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal diversity (2012) 56:1–29. doi: 10.1007/s13225-012-0187-4
CrossRef Full Text | Google Scholar
49 Gu Y, Fang Y, Wu X, Xu T, Hu T, Xu Y, et al. The emerging roles of SUMOylation in the tumor microenvironment and therapeutic implications. Exp Hematol Oncol. (2023) 12:58. doi: 10.1186/s40164-023-00420-3
CrossRef Full Text | Google Scholar
50 Rabellino A, Carter B, Konstantinidou G, Wu SY, Rimessi A, Byers LA, et al. The SUMO E3-ligase PIAS1 regulates the tumor suppressor PML and its oncogenic counterpart PML-RARA. Cancer Res. (2012) 72:2275–84. doi: 10.1158/0008-5472.CAN-11-3159
CrossRef Full Text | Google Scholar
51 Shangguan X, He J, Ma Z, Zhang W, Ji Y, Shen K, et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat Commun. (2021) 12:1812. doi: 10.1038/s41467-021-22163-7
CrossRef Full Text | Google Scholar
52 Hou G, Zhao X, Li L, Yang Q, Liu X, Huang C, et al. SUMOylation of YTHDF2 promotes mRNA degradation and cancer progression by increasing its binding affinity with m6A-modified mRNAs. Nucleic Acids Res. (2021) 49:2859–77. doi: 10.1093/nar/gkab065
CrossRef Full Text | Google Scholar
53 Yao S, Wang W, Zhou B, Cui X, Yang H, Zhang S. Monensin suppresses cell proliferation and invasion in ovarian cancer by enhancing MEK1 SUMOylation. Exp Ther Med. (2021) 22:1390. doi: 10.3892/etm
CrossRef Full Text | [Google Scholar](http://scholar.google.com/scholar_lookup?author=S+Yao&author=W+Wang&author=B+Zhou&author=X+Cui&author=H+Yang&author=S+Zhang&publication_year=2021&title=Monensin%20suppresses%20cell%20proliferation%20and%20invasion%20in%20ovarian%20cancer%20by%20enhancing%20MEK1
