The periodic table is more than just a list of elements; it’s a carefully organized chart that reflects the recurring patterns in the chemical behavior of elements. This organization is largely based on electron configuration, which dictates how elements interact with each other. Elements are grouped into families, also known as groups, based on these shared chemical properties.
Key Families in the Periodic Table
A group or family is a vertical column on the periodic table. Elements within the same group exhibit similar chemical characteristics. This similarity arises because they possess the same number of valence electrons—the electrons in the outermost shell that participate in chemical bonding. Let’s delve into some prominent families:
Alkali Metals (Group 1A or 1)
Group 1A, also known as alkali metals, is the first column on the periodic table. This family includes elements like lithium (Li), sodium (Na), and potassium (K). All alkali metals have one valence electron, making them highly reactive. They readily lose this electron to form positive ions, leading to vigorous reactions with water and halogens. Despite being metals, alkali metals are surprisingly soft and can be cut with a knife.
Alkaline Earth Metals (Group 2A or 2)
Moving to Group 2A, we find the alkaline earth metals, including beryllium (Be), magnesium (Mg), and calcium (Ca). These elements have two valence electrons and are also reactive, though less so than alkali metals. They also tend to lose electrons to form positive ions and react with nonmetals. Alkaline earth metals are harder and denser than alkali metals.
Halogens (Group 7A or 17)
Located on the right side of the periodic table, Group 7A or 17 is home to the halogens, such as fluorine (F), chlorine (Cl), and bromine (Br). Halogens are nonmetals characterized by having seven valence electrons. This electron configuration makes them highly reactive nonmetals, eager to gain one electron to achieve a stable electron configuration. They readily react with metals to form salts.
Noble Gases (Group 8A or 18)
Group 8A or 18 is occupied by the noble gases, also known as inert gases. This family includes helium (He), neon (Ne), and argon (Ar). Noble gases are unique because they have a full outer shell of valence electrons (two for helium, eight for others), making them exceptionally stable and unreactive. They exist as gases at room temperature and rarely form chemical compounds.
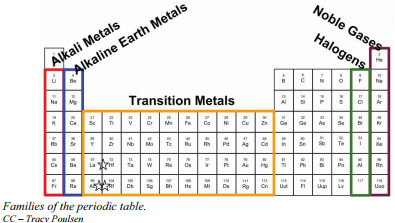 Periodic table showing group numbers 1-18 and alternative group numbers 1A-8A for main group elements, highlighting families like alkali metals, alkaline earth metals, halogens, and noble gases.
Periodic table showing group numbers 1-18 and alternative group numbers 1A-8A for main group elements, highlighting families like alkali metals, alkaline earth metals, halogens, and noble gases.
Transition Metals (Groups 3-12)
While not specifically named as “families” in the same way as main group elements, Groups 3 through 12 are collectively known as transition metals. These elements exhibit a wide range of properties and are characterized by having electrons in their d orbitals. Transition metals are typically hard, dense, and have high melting and boiling points. They are less reactive than alkali and alkaline earth metals.
Periods of the Periodic Table
In contrast to groups, periods are the horizontal rows of the periodic table. While elements in the same period do not share similar chemical properties in the same way as families, they do share the same highest energy level for their electrons. For instance, sodium (Na) and chlorine (Cl) are both in period 3, indicating their valence electrons are in the third energy level.
Conclusion
The organization of the periodic table into families is a powerful tool for understanding and predicting the chemical behavior of elements. Elements within the same family share similar electron configurations, resulting in similar chemical properties. Recognizing these families—alkali metals, alkaline earth metals, halogens, and noble gases—provides a foundational understanding of chemistry and the interactions between elements.
