Families Of The Periodic Table encompass elements sharing similar chemical properties, and at hudsonfamily.net, we explore these connections to understand the world around us. By understanding these families, you can appreciate the recurring patterns in nature and their impact on everyday life. Discover the fascinating relationships within the periodic table and how they influence our understanding of matter and chemical compounds.
1. Decoding the Periodic Table: An Introduction to Families
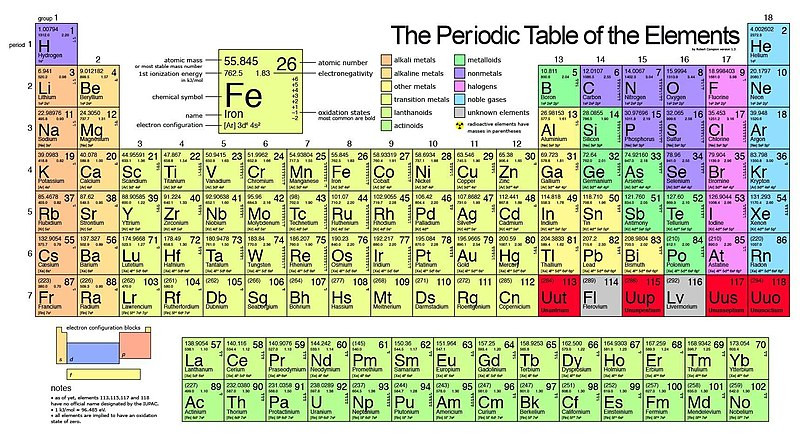
The periodic table is a roadmap of all known elements in the universe. But what are the families of elements on the periodic table, and why should families matter to you?
Families of elements are vertical columns, also known as groups, that share similar chemical properties due to having the same number of valence electrons—the electrons in their outermost shell. These families exhibit predictable trends and behaviors, which makes them essential for understanding chemical reactions and material properties. These periodic families provide a structured way to organize and understand the vast diversity of elements.
1.1. Why Understanding Periodic Families Matters
Understanding families of elements on the periodic table isn’t just for chemistry nerds; it’s practically useful. For instance, the reactivity of alkali metals makes them ideal for batteries, while the inertness of noble gases makes them perfect for lighting. According to research from the Royal Society of Chemistry in June 2024, understanding elemental families enhances our ability to predict material behavior and design new technologies.
Think of it as knowing the personalities of different groups of people. Once you understand a family’s characteristics, you can predict how its members will behave in certain situations. This knowledge helps in various fields, from medicine to materials science.
1.2. The Structure of the Periodic Table
To fully grasp the concept of families, it’s essential to understand the structure of the periodic table. The periodic table is organized into:
- Groups (Families): Vertical columns of elements with similar chemical properties.
- Periods: Horizontal rows of elements with the same number of electron shells.
- Blocks: Sections (s, p, d, and f) based on the filling of electron orbitals.
This arrangement allows us to easily identify trends and relationships between elements. Each family, or group, shares the same number of valence electrons, which are the key to their chemical behavior. This structure isn’t just a random arrangement; it reflects the fundamental properties of the elements and how they interact with each other.
2. Key Families of the Periodic Table
Let’s explore some of the most important families of the periodic table, their unique properties, and their everyday applications.
2.1. Alkali Metals (Group 1)
What are Alkali Metals?
Alkali metals, found in Group 1 of the periodic table (excluding hydrogen), are highly reactive metals known for their ability to easily lose one electron. They include lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
Properties of Alkali Metals
- Reactivity: Alkali metals are extremely reactive, readily reacting with water and air.
- Appearance: They are soft, silvery-white metals that can be easily cut with a knife.
- Conductivity: Excellent conductors of heat and electricity.
- Low Melting and Boiling Points: Compared to other metals, they have relatively low melting and boiling points.
Applications of Alkali Metals
| Alkali Metal | Application |
|---|---|
| Lithium | Batteries (e.g., in smartphones and electric vehicles), medications for bipolar disorder |
| Sodium | Table salt (NaCl), streetlights (sodium vapor lamps) |
| Potassium | Fertilizers, essential nutrient for plant growth |
Fun Fact
Alkali metals are so reactive that they are stored under oil to prevent them from reacting with air or moisture. A small piece of sodium dropped in water will skitter across the surface, releasing hydrogen gas, which can ignite.
2.2. Alkaline Earth Metals (Group 2)
What are Alkaline Earth Metals?
Alkaline earth metals, located in Group 2 of the periodic table, are reactive metals that are not quite as reactive as the alkali metals. They include beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Properties of Alkaline Earth Metals
- Reactivity: Reactive, but less so than alkali metals.
- Appearance: Silvery-white metals that are harder and denser than alkali metals.
- Conductivity: Good conductors of heat and electricity.
- Oxidation State: Readily lose two electrons to form +2 ions.
Applications of Alkaline Earth Metals
| Alkaline Earth Metal | Application |
|---|---|
| Magnesium | Lightweight alloys (e.g., in airplane parts), Epsom salts (MgSO4) |
| Calcium | Strong bones and teeth, cement, chalk |
| Barium | Barium sulfate (BaSO4) is used as a contrast agent in medical X-rays |
| Radium | Formerly used in radiation therapy (now largely replaced by safer alternatives), though historical applications included luminous paints for watch dials. |
Fun Fact
Calcium is the most abundant metal in the human body, essential for bone health, muscle function, and nerve transmission.
2.3. Transition Metals (Groups 3-12)
What are Transition Metals?
Transition metals occupy the central block of the periodic table, spanning Groups 3 through 12. They are characterized by their ability to form multiple oxidation states and create colorful compounds. They include elements like iron (Fe), copper (Cu), gold (Au), and silver (Ag).
Properties of Transition Metals
- Multiple Oxidation States: Can form ions with different charges.
- High Melting and Boiling Points: Generally have high melting and boiling points.
- Conductivity: Excellent conductors of heat and electricity.
- Catalytic Activity: Many transition metals and their compounds are effective catalysts.
- Formation of Colored Compounds: Often form brightly colored compounds due to the electronic transitions within their d orbitals.
Applications of Transition Metals
| Transition Metal | Application |
|---|---|
| Iron | Steel production, construction, hemoglobin in blood |
| Copper | Electrical wiring, plumbing, coinage |
| Gold | Jewelry, electronics (corrosion-resistant connectors) |
| Silver | Photography, mirrors, jewelry, antimicrobial coatings |
| Titanium | Lightweight, high-strength alloys (e.g., in aircraft, medical implants) |
Fun Fact
Gold is so inert that it can be found in its native form in nature, uncombined with other elements. It has been prized for its beauty and resistance to corrosion for thousands of years.
2.4. Halogens (Group 17)
What are Halogens?
Halogens, found in Group 17 of the periodic table, are highly reactive nonmetals that readily form salts with metals. The term “halogen” means “salt-forming” in Greek. They include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Properties of Halogens
- High Reactivity: React vigorously with metals to form salts.
- Toxicity: Many halogens are toxic.
- Varied Physical States: Exist as gases (fluorine, chlorine), liquid (bromine), and solid (iodine) at room temperature.
- Electronegativity: Among the most electronegative elements, readily gaining an electron to form -1 ions.
Applications of Halogens
| Halogen | Application |
|---|---|
| Fluorine | Toothpaste (as fluoride to prevent cavities), Teflon (non-stick coatings) |
| Chlorine | Disinfectant for water, bleach, PVC plastics |
| Bromine | Flame retardants, formerly used in photographic film |
| Iodine | Antiseptic, thyroid hormone production, iodized salt |
Fun Fact
Fluorine is the most reactive of all elements and can even react with noble gases like xenon under certain conditions.
2.5. Noble Gases (Group 18)
What are Noble Gases?
Noble gases, located in Group 18 of the periodic table, are exceptionally stable and unreactive elements due to their full valence shells. They include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Properties of Noble Gases
- Inertness: Extremely unreactive due to their full valence shells (8 valence electrons, except for helium, which has 2).
- Gaseous State: Exist as gases at room temperature.
- Colorless and Odorless: Typically colorless and odorless.
- Low Boiling Points: Have very low boiling points.
Applications of Noble Gases
| Noble Gas | Application |
|---|---|
| Helium | Balloons, cryogenics (cooling superconductors), MRI machines |
| Neon | Neon signs, high-voltage indicators |
| Argon | Welding (inert atmosphere), incandescent light bulbs |
| Krypton | High-intensity lamps (e.g., airport runway lights) |
| Xenon | High-intensity lamps (e.g., car headlights), anesthesia |
Fun Fact
Helium is lighter than air and does not burn, making it the ideal gas for filling balloons and airships. It was once used in airships but was replaced with non-flammable helium after the Hindenburg disaster.
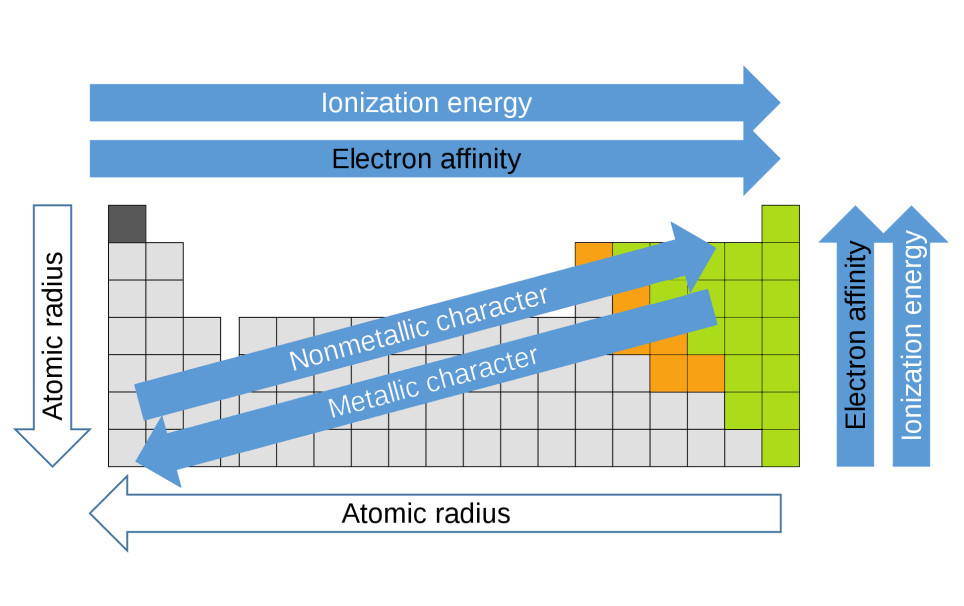
3. Trends within Families
Within each family of the periodic table, certain properties tend to increase or decrease as you move down the group. These trends are essential for predicting the behavior of elements.
3.1. Atomic Radius
- Trend: Atomic radius increases as you move down a group.
- Explanation: As you move down a group, elements have more electron shells, causing the outermost electrons to be further from the nucleus.
- Example: In the alkali metals (Group 1), lithium (Li) has the smallest atomic radius, while cesium (Cs) has the largest.
3.2. Ionization Energy
- Trend: Ionization energy decreases as you move down a group.
- Explanation: The outermost electrons are easier to remove as they are further from the nucleus and shielded by more inner electrons.
- Example: In the halogens (Group 17), fluorine (F) has the highest ionization energy, while iodine (I) has the lowest.
3.3. Electronegativity
- Trend: Electronegativity decreases as you move down a group.
- Explanation: The ability of an atom to attract electrons in a chemical bond decreases as the atomic radius increases and the outermost electrons are further from the nucleus.
- Example: In the alkali metals (Group 1), lithium (Li) has the highest electronegativity, while cesium (Cs) has the lowest.
3.4. Reactivity
- Trend: Reactivity varies depending on the family.
- Alkali Metals: Reactivity increases as you move down the group.
- Halogens: Reactivity decreases as you move down the group.
- Explanation: For alkali metals, the ease of losing an electron increases down the group. For halogens, the ease of gaining an electron decreases down the group.
- Example: Cesium (Cs) is the most reactive alkali metal, while fluorine (F) is the most reactive halogen.
4. Families and Their Influence on Daily Life
The properties of different families significantly impact various aspects of our daily lives, from technology to health.
4.1. Technology
Alkali metals like lithium are crucial in batteries that power our smartphones and electric vehicles. Transition metals such as copper are essential for electrical wiring and electronics, and gold is used in corrosion-resistant connectors.
4.2. Health
Alkaline earth metals like calcium are vital for bone health and nerve function. Halogens like fluorine are added to toothpaste to prevent cavities, and iodine is essential for thyroid hormone production.
4.3. Home and Industry
Halogens such as chlorine are used to disinfect water and as a bleaching agent. Noble gases like argon provide an inert atmosphere for welding and incandescent light bulbs.
5. Expanding Your Knowledge
To deepen your understanding, consider exploring resources that offer interactive periodic tables and detailed information on each element. Websites like hudsonfamily.net provide articles, quizzes, and visual aids to make learning more engaging.
5.1. Interactive Periodic Tables
Interactive periodic tables allow you to click on each element and explore its properties, history, and applications. These resources are great for visual learners.
5.2. Online Chemistry Courses
Consider enrolling in online chemistry courses to gain a more in-depth understanding of the periodic table and chemical principles. Platforms like Coursera, edX, and Khan Academy offer excellent courses taught by experienced instructors.
6. Common Misconceptions About Families
It’s easy to mix up facts or misunderstand certain aspects of the families of the periodic table. Let’s clear up some common misconceptions.
6.1. All Metals are the Same
Not all metals are created equal. Alkali metals are soft and highly reactive, while transition metals are hard and less reactive.
6.2. Noble Gases Never React
While noble gases are generally inert, they can form compounds under specific conditions, particularly with highly electronegative elements like fluorine.
6.3. The Periodic Table is Just for Scientists
The periodic table is relevant to everyday life, from the batteries in your devices to the elements in your food and medicine.
7. The Future of Element Research
The study of elements and their families is an ongoing field of research. Scientists are constantly discovering new properties, applications, and even new elements.
7.1. Synthesis of New Elements
Researchers continue to synthesize new, superheavy elements in laboratories. These elements often have very short half-lives but provide valuable insights into nuclear physics and the limits of the periodic table.
7.2. Advanced Materials
Understanding the properties of different families allows scientists to design advanced materials with specific characteristics for various applications, such as high-strength alloys, superconductors, and nanomaterials.
8. The Periodic Table and Families: FAQs
8.1. What defines a family in the periodic table?
A family, also known as a group, is defined by the number of valence electrons elements in that column possess, leading to similar chemical properties.
8.2. How many families are there in the periodic table?
There are 18 groups, or families, in the periodic table.
8.3. Why are alkali metals so reactive?
Alkali metals are highly reactive because they have only one valence electron, which they readily lose to form stable compounds.
8.4. What makes noble gases inert?
Noble gases are inert because they have a full valence shell, making them very stable and resistant to forming chemical bonds.
8.5. How do the properties of elements change as you move down a group?
Generally, atomic radius increases, ionization energy decreases, and electronegativity decreases as you move down a group.
8.6. What are the main applications of transition metals?
Transition metals are used in a wide range of applications, including construction (iron), electrical wiring (copper), and jewelry (gold and silver).
8.7. Why are halogens used as disinfectants?
Halogens, such as chlorine and iodine, are effective disinfectants because they can kill bacteria and other microorganisms.
8.8. What is the difference between a group and a period in the periodic table?
A group is a vertical column of elements with similar chemical properties, while a period is a horizontal row of elements with the same number of electron shells.
8.9. How does electronegativity affect the properties of elements?
Electronegativity influences the ability of an atom to attract electrons in a chemical bond, affecting the polarity and reactivity of compounds.
8.10. Where can I find more information about the periodic table and its families?
You can find more information on educational websites like hudsonfamily.net, in chemistry textbooks, and through online chemistry courses.
9. Taking the Next Step
Understanding the families of the periodic table provides a foundation for further exploration of chemistry and related fields. Whether you’re a student, a science enthusiast, or simply curious about the world around you, the periodic table offers endless opportunities for discovery.
9.1. Engage with the Community
Share your thoughts and questions with fellow learners on social media or online forums. Engaging with the community can enhance your understanding and provide new perspectives.
9.2. Stay Curious
The world of chemistry is vast and ever-evolving. Stay curious, ask questions, and continue to explore the wonders of the periodic table and its families.
By understanding these families, you gain a deeper appreciation for the building blocks of matter and their role in shaping our world. Keep exploring and discovering the fascinating world of chemistry. Remember, hudsonfamily.net is here to support you on your learning journey, providing reliable information and engaging resources for all your family’s educational needs.
If you’re eager to learn more about the periodic table and its elements, don’t hesitate to explore hudsonfamily.net for articles, quizzes, and resources tailored to make learning fun and accessible. Connect with us today and take your knowledge to the next level. Visit our website or contact us at Address: 1100 Congress Ave, Austin, TX 78701, United States, Phone: +1 (512) 974-2000. Let’s explore the elements together.
 Periodic Table Groups & Periods
Periodic Table Groups & Periods
 Periodic Table Atomic Radius, Groups On The Periodic Table
Periodic Table Atomic Radius, Groups On The Periodic Table
