Periodic table family names are essential knowledge for understanding chemistry and the properties of elements. This comprehensive guide, brought to you by hudsonfamily.net, will explore these families in detail, providing you with a clear and accessible understanding. Delve into the element arrangement, characteristics, and significance of these chemical families to simplify family science and element behavior, thus enhancing knowledge and appreciation.
1. What is the Periodic Table and Why is it Organized into Families?
The periodic table organizes elements based on their atomic number and recurring chemical properties, and it is divided into vertical columns called groups and horizontal rows called periods, but a family is a group of elements with similar properties, the families on the periodic table are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble gases, lanthanides and actinides. This arrangement helps in predicting element behavior and understanding chemical reactions.
- The periodic table is a fundamental tool in chemistry, organizing elements in a way that reflects their properties and behaviors.
- The modern periodic table is arranged by increasing atomic number, which corresponds to the number of protons in an atom’s nucleus.
- This arrangement reveals periodic trends, such as electronegativity and ionization energy, which are critical to understanding how elements interact.
- The periodic table is more than just a list; it’s a roadmap for understanding the chemical universe, guiding predictions and discoveries.
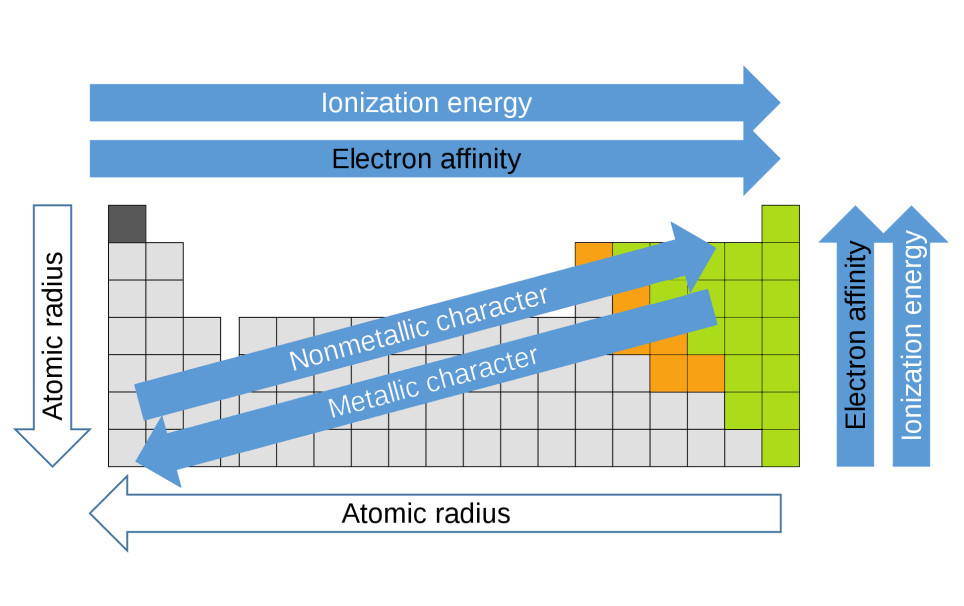 Periodic table showing atomic radius and groups
Periodic table showing atomic radius and groups
1.1 How Does Grouping Elements into Families Simplify the Study of Chemistry?
Grouping elements into families simplifies the study of chemistry because elements within a family share similar chemical properties and behaviors, making it easier to predict their reactions and characteristics. This classification reduces the complexity of studying each element individually.
- Families, also known as groups, are vertical columns on the periodic table, and each family contains elements with similar valence electron configurations.
- Elements in the same family tend to exhibit similar chemical properties, such as reactivity and bonding behavior.
- Understanding the properties of one element in a family provides insights into the properties of other elements in the same family.
- This simplification allows chemists and students to focus on the overarching trends and characteristics of families rather than memorizing individual element properties.
1.2 What Are the Key Differences Between Groups, Periods, and Families in the Periodic Table?
Groups are vertical columns, periods are horizontal rows, and families are sets of elements with similar chemical properties. Groups share the same number of valence electrons, periods share the same number of electron shells, and families share similar reactivity and behavior.
| Feature | Groups | Periods | Families |
|---|---|---|---|
| Orientation | Vertical columns | Horizontal rows | Groupings of elements with similar properties, which can span multiple groups |
| Basis of division | Number of valence electrons | Number of electron shells | Chemical properties |
| Property Shared | Similar chemical properties | Gradual change in properties across the row | Similar chemical behavior |
| Examples | Alkali metals, halogens, noble gases | Period 1, Period 2, Period 3 | Alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble gases, lanthanides, and actinides. |
| Usefulness | Predicting chemical reactions | Observing trends in element properties | Simplifies the study of elements by categorizing them based on shared characteristics, aids in predicting element behavior and understanding chemical reactions, useful for teaching and learning chemistry, allows for comparative analysis of element properties, helps in identifying patterns and relationships in the periodic table, essential for chemical research and development, and useful for classifying elements in different applications. |
2. What are the Alkali Metals (Group 1)?
Alkali metals, found in Group 1 of the periodic table, are highly reactive metals that readily lose one electron to form positive ions. They include lithium, sodium, potassium, rubidium, cesium, and francium.
- Alkali metals are characterized by their high reactivity, especially with water and air.
- They are soft, silvery metals that can be easily cut with a knife.
- Due to their reactivity, alkali metals are never found in their free state in nature.
- They are essential in various industrial processes and biological functions, such as nerve impulse transmission (sodium and potassium).
2.1 What Makes Alkali Metals Highly Reactive?
Alkali metals are highly reactive due to their single valence electron, which they readily lose to achieve a stable electron configuration. This low ionization energy makes them strong reducing agents, easily forming positive ions and reacting with other elements.
- Alkali metals have the lowest ionization energies in their respective periods, which means they require very little energy to lose their single valence electron.
- This makes them excellent reducing agents, readily donating their electron to other elements in chemical reactions.
- The resulting positive ions are very stable, as they have achieved a noble gas electron configuration.
- Their high reactivity explains why they are always found in compounds and never in their elemental form in nature.
2.2 What are Some Common Uses and Applications of Alkali Metals?
Alkali metals have diverse applications: lithium in batteries and pharmaceuticals, sodium in streetlights and table salt, potassium in fertilizers, and cesium in atomic clocks. Their unique properties make them essential in various industries and technologies.
| Alkali Metal | Application |
|---|---|
| Lithium | Lithium is used in rechargeable batteries for electronic devices, electric vehicles, and energy storage systems, it is used in the treatment of bipolar disorder, acting as a mood stabilizer. It is used in the production of lubricants, ceramics, and glasses, it is also used in air purification systems. |
| Sodium | Sodium is a component of table salt (sodium chloride), which is essential for human health and food preservation, it is used in the production of various chemicals, including sodium hydroxide (lye), used in soap and paper manufacturing, sodium vapor lamps are used for street lighting due to their energy efficiency, sodium is used as a coolant in nuclear reactors to transfer heat, it is involved in nerve impulse transmission and muscle function in living organisms, it is used as a reducing agent in various chemical reactions. |
| Potassium | Potassium is a key component of fertilizers, promoting plant growth and crop yield, it is essential for nerve function, muscle contraction, and maintaining fluid balance in living organisms, potassium chloride is used as a salt substitute for individuals with high blood pressure, potassium is used in the production of soaps and detergents, potassium iodide is added to table salt to prevent iodine deficiency, potassium is used in the manufacturing of glass and ceramics, it is also used in the production of gunpowder and fireworks, involved in maintaining proper heart function, potassium compounds are used in photography for developing images, and it helps regulate blood pressure and prevent hypertension. |
| Rubidium | Rubidium is used in atomic clocks, which are highly accurate timekeeping devices, it is used in some specialized electronic devices, rubidium is used in medical imaging techniques such as PET scans, it is also used in vacuum tubes. |
| Cesium | Cesium is used in atomic clocks, which are highly accurate timekeeping devices used in GPS systems and telecommunications, cesium is used in photoelectric cells, which convert light into electricity, cesium is used in some specialized electronic devices, it is used in the production of high-intensity blue light, cesium is also used in vacuum tubes, plays a role in maintaining blood sugar levels, and it also plays a role in muscle function. |
| Francium | Francium is highly radioactive and has no significant practical applications due to its rarity and instability, it is primarily used for scientific research purposes, francium’s radioactivity makes it useful in studies related to atomic structure and nuclear physics, it is used in understanding radioactive decay processes, francium is used in the study of fundamental properties of elements, it is also used in the study of rare elements. |
3. What are the Alkaline Earth Metals (Group 2)?
Alkaline earth metals, located in Group 2, are reactive metals that readily lose two electrons to form positive ions. They include beryllium, magnesium, calcium, strontium, barium, and radium.
- Alkaline earth metals are less reactive than alkali metals but still readily form compounds.
- They are harder, denser, and have higher melting points compared to alkali metals.
- Calcium and magnesium are essential for biological functions, such as bone formation and muscle function.
- These metals are used in various applications, from construction materials to medical treatments.
3.1 How Do Alkaline Earth Metals Differ From Alkali Metals in Terms of Reactivity?
Alkaline earth metals are less reactive than alkali metals because they have two valence electrons that require more energy to remove compared to the single valence electron of alkali metals. While both groups are reactive, alkaline earth metals react more slowly.
- Alkaline earth metals have higher ionization energies than alkali metals, requiring more energy to lose their two valence electrons.
- This makes them less prone to forming positive ions compared to alkali metals.
- Alkaline earth metals also form stronger bonds with other elements due to their higher charge density.
- As a result, they react more slowly and less vigorously than alkali metals.
3.2 What are Some Important Roles of Alkaline Earth Metals in Biological Systems and Industry?
Alkaline earth metals play crucial roles in biological systems and industry: calcium is vital for bone and teeth formation and muscle function, magnesium is essential for photosynthesis and enzyme activity, and barium is used in medical imaging.
| Element | Biological Role | Industrial Application |
|---|---|---|
| Beryllium | No known biological role. | Used in alloys for aerospace applications, high-strength materials, and nuclear reactors. |
| Magnesium | Essential for photosynthesis in plants, enzyme activity, and nerve and muscle function in animals. | Used in lightweight alloys for aircraft, automotive parts, and electronic devices; also used in Epsom salts (magnesium sulfate) for medicinal purposes. |
| Calcium | Vital for bone and teeth formation, muscle function, nerve transmission, and blood clotting. | Used in cement and mortar for construction, as a dietary supplement, and in the production of lime. |
| Strontium | No known biological role, but strontium compounds can be absorbed into bone. | Used in fireworks to produce a red color, in some luminous paints, and in the production of strontium carbonate for various applications. |
| Barium | No known biological role; barium compounds are toxic. | Barium sulfate is used as a contrast agent in medical X-rays to enhance imaging of the digestive system, also used in the production of rubber and plastics, and in oil well drilling. |
| Radium | No known biological role; radium is radioactive and highly toxic. | Formerly used in luminous paints for watch dials and medical treatments for cancer, but now largely replaced due to its radioactivity. Used in some industrial radiography applications. |
4. What are the Transition Metals (Groups 3-12)?
Transition metals, found in Groups 3-12, are characterized by their ability to form multiple oxidation states and colorful compounds. They include elements like iron, copper, gold, and silver.
- Transition metals are known for their high strength, hardness, and ability to conduct electricity and heat.
- They often form complex ions and act as catalysts in chemical reactions.
- Many transition metals are essential for life, such as iron in hemoglobin and zinc in enzymes.
- They are widely used in construction, electronics, and jewelry.
4.1 What Unique Properties Define Transition Metals?
Transition metals are defined by their ability to form multiple oxidation states due to the presence of partially filled d-orbitals. This leads to colorful compounds, catalytic activity, and the formation of complex ions.
- The partially filled d-orbitals allow transition metals to lose electrons from different energy levels, resulting in multiple oxidation states.
- Their ability to act as catalysts stems from their ability to adsorb reactants onto their surface and facilitate chemical reactions.
- Transition metals form complex ions by bonding with ligands, creating colorful solutions and compounds.
- These unique properties make transition metals essential in various industrial and biological processes.
4.2 How Are Transition Metals Used in Everyday Life and Industries?
Transition metals are integral to everyday life and industries: iron is used in construction, copper in electrical wiring, titanium in aerospace, and gold and silver in jewelry. Their versatility makes them essential in numerous applications.
| Transition Metal | Use in Everyday Life | Industrial Application |
|---|---|---|
| Iron (Fe) | Used in cookware, appliances, and tools, iron is essential for hemoglobin in red blood cells, carrying oxygen throughout the body, iron supplements are commonly taken to prevent or treat iron deficiency anemia, iron is a component of many vitamins and mineral supplements, iron compounds are used as pigments in paints, inks, and cosmetics, iron is present in various forms in jewelry, such as stainless steel, iron is a component of certain types of batteries, used in electrical transformers and motors, involved in various enzymatic reactions, iron is important for proper growth and development. | Used in the production of steel for construction, automotive, and manufacturing industries, iron is a catalyst in the Haber-Bosch process for ammonia production, iron is used in the production of magnets and magnetic materials, used in the manufacturing of various chemicals, iron is used in the production of pigments and dyes, used in the manufacturing of machinery and equipment, iron is used in the production of tools and implements, iron is a key component in the production of various metal alloys, iron is used in the production of pipes and tubing, iron is used in the production of railway tracks and rolling stock. |
| Copper (Cu) | Used in electrical wiring, plumbing pipes, and cookware, copper is an essential nutrient for human health, copper is used in jewelry and decorative items, copper is a component of many alloys, such as brass and bronze, copper is used in roofing and guttering, copper is used in coins, copper compounds are used as fungicides and algaecides, copper is used in heat exchangers and radiators, copper is a component of some vitamins and mineral supplements, copper is used in musical instruments. | Copper is used in the production of electrical wiring, cables, and electronic components, copper is used in the production of plumbing pipes and fittings, used in the production of alloys such as brass and bronze, copper is used in the production of heat exchangers and radiators, copper is used in the production of roofing and guttering, copper is used in the production of coins, copper is used in the production of electrical motors and generators, copper is used in the production of telecommunications equipment, copper is used in the production of marine hardware, copper is used in the production of industrial machinery. |
| Titanium (Ti) | Used in eyeglass frames, watches, and jewelry, titanium dioxide is used in sunscreen and cosmetics to protect against UV radiation, titanium is used in some medical implants, titanium is used in sporting equipment such as golf clubs and tennis rackets, titanium is used in bicycle frames, titanium is used in dental implants, titanium dioxide is used as a pigment in paints, plastics, and paper, titanium is used in kitchen utensils and cookware, titanium is used in mobile phones and laptops, titanium is used in watches. | Titanium is used in aerospace engineering for aircraft and spacecraft components, titanium is used in the production of medical implants such as hip replacements and dental implants, titanium is used in chemical processing equipment due to its corrosion resistance, titanium is used in the construction of offshore oil platforms, titanium is used in the production of sporting equipment such as golf clubs and tennis rackets, titanium is used in the construction of bridges, titanium is used in the production of automotive components, titanium is used in the production of armor plating, titanium is used in the construction of naval vessels, titanium is used in the production of desalination plants. |
| Gold (Au) | Used in jewelry, coins, and decorative items, gold is used in electronics for connectors and circuitry, gold is used in dentistry for fillings and crowns, gold is used in some medications, gold is used in decorative accents for architecture, gold is used in collectibles such as coins and bars, gold is used in commemorative items, gold is used in trophies and awards, gold is used in conductive inks for printed electronics, gold is used in jewelry. | Gold is used in the production of electronic components such as connectors and circuitry, gold is used in the production of jewelry, gold is used as a store of value and investment, gold is used in the production of dental fillings and crowns, gold is used in the production of chemical catalysts, gold is used in the production of corrosion-resistant coatings, gold is used in the production of heat-reflective coatings, gold is used in the production of radiation shielding, gold is used in the production of laboratory equipment, gold is used in the production of spacecraft components. |
| Silver (Ag) | Used in jewelry, silverware, and mirrors, silver is used in photography for developing images, silver is used in electronics for connectors and circuitry, silver is used in medicine for its antimicrobial properties, silver is used in water purification systems, silver is used in some clothing and textiles for odor control, silver is used in some food packaging for its antimicrobial properties, silver is used in musical instruments, silver is used in commemorative items, silver is used in jewelry. | Silver is used in the production of electronic components such as connectors and circuitry, silver is used in the production of jewelry, silver is used in photography for developing images, silver is used as a store of value and investment, silver is used in the production of mirrors, silver is used in the production of antimicrobial coatings, silver is used in the production of batteries, silver is used in the production of chemical catalysts, silver is used in the production of dental fillings, silver is used in the production of solar panels. |
5. What are the Lanthanides and Actinides (Inner Transition Metals)?
Lanthanides and actinides, also known as inner transition metals, are located in the f-block of the periodic table. Lanthanides include elements like cerium and neodymium, while actinides include uranium and plutonium.
- Lanthanides are used in magnets, lasers, and phosphors, while actinides are primarily known for their radioactivity and use in nuclear energy.
- These elements have unique electronic configurations due to their filling f-orbitals.
- The actinides are all radioactive, and some are synthetic, not found naturally on Earth.
- They have important applications in various high-tech industries and nuclear medicine.
5.1 Why Are Lanthanides and Actinides Called Inner Transition Metals?
Lanthanides and actinides are called inner transition metals because they fill the f-orbitals, which lie within the electron shells of the transition metals. This unique electronic configuration gives them distinct properties and separates them from the main transition metal series.
- The filling of the f-orbitals occurs in the inner electron shells, which is why they are referred to as “inner.”
- This electronic configuration affects their chemical behavior, leading to similar properties within each series.
- Lanthanides and actinides are placed separately at the bottom of the periodic table to avoid making the table too wide.
- Their unique properties make them valuable in various specialized applications.
5.2 What Are the Primary Uses of Lanthanides and Actinides in Modern Technology?
Lanthanides are used in magnets for electronics, lasers for medical and industrial applications, and phosphors for displays. Actinides are primarily used in nuclear energy, with uranium and plutonium being key components in nuclear reactors and weapons.
| Element | Technological Use |
|---|---|
| Cerium | Cerium is used as a polishing compound for glass lenses and mirrors, it is used in catalytic converters in automobiles to reduce emissions, cerium is used in cigarette lighters to create sparks, cerium is used in the production of mischmetal, an alloy used in lighter flints, cerium compounds are used as catalysts in various chemical reactions, cerium oxide is used in oxygen sensors, cerium is used in carbon arc lamps, cerium is used in the production of magnets, cerium compounds are used in textile dyeing, and cerium compounds are used in the production of ceramics. |
| Neodymium | Neodymium is used in powerful magnets for electronics, wind turbines, and electric vehicles, neodymium is used in lasers for medical and industrial applications, neodymium is used in coloring glass and ceramics, neodymium is used in the production of speakers and headphones, neodymium is used in lighting applications, neodymium compounds are used as catalysts in chemical reactions, neodymium is used in magnetic resonance imaging (MRI) machines, neodymium is used in the production of guitar pickups, neodymium is used in the production of high-intensity lamps, and neodymium compounds are used in the production of synthetic rubber. |
| Uranium | Uranium is used as fuel in nuclear reactors to generate electricity, uranium is used in the production of nuclear weapons, uranium is used in some medical treatments for cancer, uranium is used in geological dating, uranium is used in some industrial applications for its density, uranium compounds are used as pigments in ceramics, uranium is used in radiation shielding, uranium is used in ballast for ships and aircraft, uranium compounds are used in photography, uranium compounds are used in the production of colored glass. |
| Plutonium | Plutonium is used in the production of nuclear weapons, plutonium is used as fuel in nuclear reactors, plutonium is used in pacemakers for long-term power, plutonium is used in space exploration for radioisotope thermoelectric generators (RTGs), plutonium is used in some research applications, plutonium compounds are used in nuclear batteries, plutonium is used in the production of heat sources for spacecraft, plutonium compounds are used in the production of advanced research reactors, plutonium compounds are used in the production of materials for nuclear fusion research, and plutonium compounds are used in the production of nuclear medicine isotopes. |
6. What are the Metalloids (Semi-metals)?
Metalloids, also known as semi-metals, possess properties of both metals and non-metals. They include boron, silicon, germanium, arsenic, antimony, and tellurium.
- Metalloids are semiconductors, meaning they can conduct electricity under certain conditions.
- They are crucial in the electronics industry, particularly in the production of computer chips and solar panels.
- Their properties can be altered by adding impurities, a process known as doping.
- Metalloids are versatile elements with applications in various fields.
6.1 How Do Metalloids Exhibit Properties of Both Metals and Non-metals?
Metalloids exhibit properties of both metals and non-metals by having intermediate electronegativity and ionization energy values. This allows them to behave as semiconductors, conducting electricity under certain conditions, and forming alloys with metals while also forming covalent bonds with non-metals.
- Their electronegativity and ionization energy values fall between those of metals and non-metals, leading to their unique properties.
- Metalloids can act as semiconductors because their electrical conductivity increases with temperature or when impurities are added.
- They form alloys with metals, enhancing their strength and corrosion resistance.
- Metalloids also form covalent bonds with non-metals, creating compounds with diverse properties.
6.2 What Makes Metalloids Essential in the Electronics Industry?
Metalloids are essential in the electronics industry due to their semiconducting properties, particularly silicon’s use in computer chips and solar panels. Their ability to control electrical conductivity makes them ideal for creating transistors, diodes, and other electronic components.
- Silicon is the most widely used metalloid in the electronics industry due to its abundance and semiconducting properties.
- Doping silicon with impurities allows precise control over its electrical conductivity, enabling the creation of transistors and diodes.
- These components are the building blocks of computer chips, microprocessors, and integrated circuits.
- Metalloids are also used in solar panels to convert sunlight into electricity, contributing to renewable energy production.
7. What are the Halogens (Group 17)?
Halogens, located in Group 17, are highly reactive non-metals that readily gain one electron to form negative ions. They include fluorine, chlorine, bromine, iodine, and astatine.
- Halogens are found in all three states of matter at room temperature: gas (fluorine and chlorine), liquid (bromine), and solid (iodine and astatine).
- They are used in disinfectants, lighting, and the production of various chemicals.
- Their reactivity makes them effective sanitizers and bleaching agents.
- Halogens are essential in numerous industrial and household applications.
7.1 What Explains the High Reactivity of Halogens?
The high reactivity of halogens is due to their electron configuration, which requires only one additional electron to achieve a stable, noble gas configuration. This strong electron affinity makes them highly reactive, readily forming negative ions.
- Halogens have seven valence electrons and a strong tendency to gain one more electron to complete their octet.
- This results in a high electron affinity, meaning they release a significant amount of energy when gaining an electron.
- Their high electronegativity also contributes to their reactivity, as they strongly attract electrons in chemical bonds.
- As a result, halogens readily react with metals to form salts and with non-metals to form various compounds.
7.2 What Are Some Common Uses of Halogens in Disinfection and Industry?
Halogens are widely used in disinfection and industry: chlorine is used to sanitize water and bleach fabrics, iodine is used as an antiseptic, and fluorine is used in toothpaste to prevent tooth decay. Their reactivity makes them effective in various applications.
| Halogen | Use in Disinfection | Use in Industry |
|---|---|---|
| Fluorine | Fluoride is added to drinking water and toothpaste to prevent tooth decay, it also helps to kill bacteria in water, fluoride is used to clean and disinfect dental equipment, fluorine is used as a disinfectant in some industrial processes, fluorine compounds are used to sterilize medical equipment, fluorine is used as a disinfectant in some cleaning products, and fluorine is used in some mouthwashes. | Fluorine is used in the production of Teflon, a non-stick coating for cookware, fluorine is used in the production of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), used as refrigerants, fluorine is used in the production of uranium hexafluoride, a compound used in uranium enrichment, fluorine is used in the production of various pharmaceuticals, fluorine is used in the production of high-performance polymers, and fluorine is used in the production of semiconductors. |
| Chlorine | Chlorine is used to disinfect drinking water, swimming pools, and sewage, used to sanitize surfaces in hospitals and food processing plants, chlorine bleach is used to disinfect household surfaces and laundry, used to kill bacteria and viruses in water treatment facilities, chlorine dioxide is used to disinfect air ducts and HVAC systems, chlorine is used in wound cleaning solutions, and chlorine is used in some hand sanitizers. | Chlorine is used in the production of polyvinyl chloride (PVC) plastics, chlorine is used in the production of paper and textiles, used in the production of various chemicals, including hydrochloric acid and bleach, chlorine is used in the production of pesticides and herbicides, chlorine is used in the production of pharmaceuticals, chlorine is used in the production of dyes and pigments, and chlorine is used in the production of solvents. |
| Bromine | Bromine is used as a disinfectant in swimming pools and hot tubs, bromine is used in the production of sanitizers for industrial water treatment, bromine compounds are used as disinfectants in some cleaning products, bromine is used in some medical applications to disinfect wounds, bromine is used to disinfect laboratory equipment, bromine is used to disinfect photographic equipment, bromine is used to disinfect hospital equipment. | Bromine is used in the production of flame retardants for plastics, textiles, and electronics, bromine is used in the production of pharmaceuticals, bromine is used in the production of agricultural chemicals, bromine is used in the production of photographic chemicals, bromine is used in the production of dyes, bromine is used in the production of oil and gas drilling fluids, and bromine is used in the production of rubber. |
| Iodine | Iodine is used as an antiseptic for cleaning wounds and preventing infection, iodine is used in water purification tablets for emergency water treatment, iodine is used in surgical scrubs to disinfect skin before surgery, iodine is used in some hand sanitizers, iodine is used to disinfect surfaces in hospitals, iodine is used to disinfect medical equipment, iodine is used to disinfect laboratory equipment. | Iodine is used in the production of pharmaceuticals, iodine is used in the production of dyes, iodine is used in the production of photographic chemicals, iodine is used in the production of animal feed supplements, iodine is used in the production of catalysts, iodine is used in the production of batteries, iodine is used in the production of iodized salt, iodine is used in the production of LCD screens, iodine is used in the production of semiconductors. |
8. What are the Noble Gases (Group 18)?
Noble gases, located in Group 18, are inert gases with complete valence shells, making them highly stable and unreactive. They include helium, neon, argon, krypton, xenon, and radon.
- Noble gases are used in lighting, insulation, and specialized applications due to their inertness.
- They have low boiling points and exist as monatomic gases at room temperature.
- Their stability is due to their full valence electron shells, making them resistant to forming chemical bonds.
- Noble gases are essential in various scientific and industrial applications.
8.1 Why Are Noble Gases Considered Inert or Unreactive?
Noble gases are considered inert or unreactive because they have a complete valence shell with eight electrons (except for helium, which has two), making them stable and resistant to forming chemical bonds. This full electron configuration results in minimal interaction with other elements.
- Their full valence shells give them a stable electron configuration, minimizing their tendency to gain, lose, or share electrons.
- Noble gases have high ionization energies and low electron affinities, indicating their resistance to forming ions.
- They have very low electronegativity values, meaning they do not strongly attract electrons in chemical bonds.
- As a result, noble gases exist as monatomic gases and rarely form compounds under normal conditions.
8.2 How Are Noble Gases Used in Lighting and Other Specialized Applications?
Noble gases are used in lighting, insulation, and other specialized applications due to their unique properties: helium in balloons and MRI machines, neon in advertising signs, argon in welding and incandescent light bulbs, and xenon in high-intensity lamps.
| Noble Gas | Use in Lighting | Other Specialized Applications |
|---|---|---|
| Helium | Helium is not used in lighting applications due to its low density and lack of chemical reactivity, helium is used in balloons for lifting and buoyancy, helium is used in cryogenics for cooling superconducting magnets, helium is used as a shielding gas for welding, helium is used in leak detection, helium is used in weather balloons for atmospheric research, helium is used in MRI machines for cooling superconducting magnets, helium is used in the production of semiconductors, and helium is used in the calibration of scientific instruments. | Helium is used as a coolant for superconducting magnets in MRI machines, helium is used as a lifting gas for balloons and airships, helium is used in leak detection systems, helium is used as a shielding gas for welding, helium is used as a carrier gas in gas chromatography, helium is used as a breathing gas for deep-sea diving, helium is used as a coolant for nuclear reactors, helium is used in the production of semiconductors, and helium is used in the calibration of scientific instruments. |
| Neon | Neon is used in neon signs for advertising and decorative purposes, neon produces a bright red-orange light when electricity is passed through it, neon is used in plasma TVs, neon is used in some types of lasers, neon is used in some types of voltage testers, neon is used in some types of indicator lights, neon is used in some types of surge protectors, neon is used in some types of cold cathode lamps, and neon is used in some types of decorative lighting. | Neon is used in cryogenic refrigeration, neon is used in high-voltage indicators, neon is used in lightning arrestors, neon is used in vacuum tubes, neon is used in wave meters, neon is used in Geiger counters, neon is used in spark plugs, neon is used in high-energy physics experiments, neon is used in the production of semiconductors, and neon is used in plasma research. |
| Argon | Argon is used in incandescent light bulbs to prevent the filament from oxidizing, argon is used in fluorescent light bulbs, argon is used in welding torches, argon is used in plasma cutting torches, argon is used in laser cutting torches, argon is used in some types of neon signs, argon is used in some types of decorative lighting, argon is used in some types of voltage testers, and argon is used in some types of indicator lights. | Argon is used as a shielding gas for welding, argon is used in the production of titanium, argon is used in the preservation of historical documents, argon is used in the production of semiconductors, argon is used in the production of light bulbs, argon is used in the production of lasers, argon is used in the production of plasma displays, argon is used in the production of metal alloys, argon is used in the production of scientific instruments, and argon is used in the |

