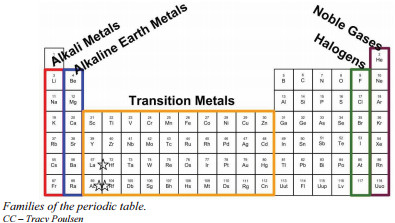
The periodic table is organized in a way that groups elements with similar chemical behaviors together. These groupings are known as families or groups. This organization isn’t arbitrary; it directly reflects the electron configurations of the elements, which dictate how they react chemically.
Understanding Families in the Periodic Table
Dmitri Mendeleev, the creator of the periodic table, structured it so that elements exhibiting the most similar properties were placed in the same vertical column. These vertical columns are what we call groups or families. The reason elements within the same family share similar chemical behavior boils down to their valence electrons – the electrons in the outermost shell of an atom.
For instance, consider Group 1A, also known as the alkali metals. Every element in this group, such as lithium (Li), sodium (Na), and potassium (K), possesses one valence electron. This shared electron configuration is why alkali metals are all highly reactive and form compounds with other elements in similar ratios and with comparable properties. Their reactivity is so pronounced that they are not found in nature in their elemental form. Interestingly, despite being metals, alkali metals are remarkably soft and can be easily cut with a knife.
Group 2A is another notable family, the alkaline earth metals, including beryllium (Be), magnesium (Mg), and calcium (Ca). Like alkali metals, alkaline earth metals exhibit similar chemical properties due to their analogous valence electron configurations – they all have two valence electrons. While still reactive, they are generally less reactive than alkali metals.
Halogens, found in Group 7A (or Group 17 in another numbering system), represent a family of highly reactive nonmetals. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) are halogens. They readily react with metals to form salts.
Finally, Group 8A, the noble gases, also known as the inert gases, includes helium (He), neon (Ne), and argon (Ar). These elements are characterized by their exceptional lack of reactivity. They rarely form chemical compounds, a property stemming from their full outer electron shells, which we will explore in more detail when discussing chemical bonding. Noble gases are also all gases at room temperature.
 Two common numbering systems for groups on the periodic table, showing both 1-18 and 1A-8A conventions.
Two common numbering systems for groups on the periodic table, showing both 1-18 and 1A-8A conventions.
It’s worth noting that there are two common systems for numbering groups in the periodic table. One system uses numbers 1-18 sequentially across the s, p, and d blocks. In this system, alkali metals (Group 1A) are Group 1, alkaline earth metals (Group 2A) are Group 2, halogens (Group 7A) are Group 17, and noble gases (Group 8A) are Group 18. The other system, often used for main group elements, uses numbers 1A-8A. It’s crucial to be familiar with both to navigate different periodic tables effectively and to quickly determine the number of valence electrons for main group elements regardless of the numbering system used.
Periods of the Periodic Table
Beyond families, the periodic table also has periods, which are the horizontal rows. The period number an element is in corresponds to the energy level of its valence electrons. For example, sodium (Na) and magnesium (Mg) are in Period 3, indicating their valence electrons are in the third energy level. Similarly, astatine (At) and radon (Rn) in Period 6 have valence electrons in the sixth energy level. Periods, however, do not share similar chemical properties like families do; instead, properties tend to change gradually across a period.
Summary
- Vertical columns in the periodic table are called groups or families. Elements in the same family exhibit similar chemical behaviors.
- This similarity in chemical properties within a family arises because family members have the same number of valence electrons.
- Horizontal rows in the periodic table are known as periods, and they indicate the energy level of valence electrons.
Vocabulary
- Group (family): A vertical column in the periodic table.
- Alkali metals: Group 1A of the periodic table.
- Alkaline earth metals: Group 2A of the periodic table.
- Halogens: Group 7A of the periodic table.
- Noble gases: Group 8A of the periodic table.
- Transition elements: Groups 3 to 12 of the periodic table.
