The periodic table is more than just a chart of elements; it’s a system that organizes elements based on their chemical properties. These properties are intrinsically linked to the electron configuration of the atoms. Elements are arranged into vertical columns known as groups or families, and horizontal rows called periods. The grouping is not arbitrary; elements within the same family exhibit similar chemical behaviors. This similarity arises because they share the same number of valence electrons, which are the electrons in the outermost shell of an atom and are primarily responsible for how an element interacts with other elements.
Understanding Families and Electron Configuration
The foundation of the periodic table’s structure lies in the chemical behavior of elements. Historically, elements were grouped together based on observed similarities in their reactions and compound formation. Dmitri Mendeleev, in his groundbreaking work, arranged the periodic table so that elements with the most alike properties were placed in the same vertical columns – the groups or families. This arrangement, based on chemical reactivity, directly corresponds to the electron configurations of the elements.
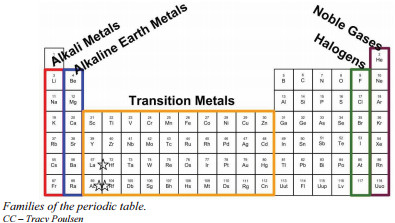
Consider Group 1A, also known as the alkali metals. Each element in this family – lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) – possesses a single valence electron. This shared electron configuration is the root cause of their similar chemical behavior. Alkali metals are all highly reactive, readily losing this one valence electron to form positive ions with a +1 charge. They react vigorously with water, halogens, and oxygen, forming compounds with comparable ratios and properties across the family. Mendeleev recognized these shared traits and placed them together. Despite the general hardness associated with metals, alkali metals are surprisingly soft, often soft enough to be cut with a knife.
 Alkali Metals in Periodic Table
Alkali Metals in Periodic Table
Similarly, Group 2A, the alkaline earth metals – beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) – display consistent properties due to their similar valence electron configurations. Each alkaline earth metal atom has two valence electrons. They are also reactive, though generally less so than alkali metals, and form ions with a +2 charge.
This pattern of shared electron configurations leading to similar chemical properties extends across other significant families in the periodic table.
Halogens: The Reactive Nonmetals
Group 7A (or Group 17 in the 1-18 numbering system), known as the halogens, includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Halogens are characterized as highly reactive nonmetals. They have seven valence electrons, needing only one more electron to achieve a stable, full outer electron shell. This electron deficiency makes them potent oxidizing agents and explains their reactivity with many metals and nonmetals.
Noble Gases: The Inert Elements
In stark contrast to the highly reactive halogens and alkali metals, Group 8A (or Group 18) houses the noble gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Noble gases are renowned for their inertness; they are exceptionally unreactive and rarely form chemical compounds. This lack of reactivity stems from their electron configurations: with the exception of helium, noble gases have a full complement of eight valence electrons (helium has a full outer shell of two). This stable electron arrangement makes them chemically satisfied and disinclined to gain, lose, or share electrons. Furthermore, all noble gases exist as gases at room temperature.
Transition Metals: The Diverse Middle
Between Group 2A and Group 3A lie the transition metals, encompassing Groups 3 to 12 of the periodic table. These elements exhibit a wide range of properties and behaviors, largely due to the involvement of their d electrons in bonding. Transition metals are typically hard, have high melting and boiling points, and are good conductors of heat and electricity. They are also known for forming colored compounds and exhibiting multiple oxidation states. Examples of transition metals include iron (Fe), copper (Cu), gold (Au), and silver (Ag).
Periods and Energy Levels
While groups highlight families with similar chemical behaviors, periods are the horizontal rows in the periodic table. Moving across a period from left to right signifies the filling of electron shells at the same principal energy level. For instance, sodium (Na) and magnesium (Mg) are both in period 3, indicating that their valence electrons are in the third energy level (n=3). Similarly, astatine (At) and radon (Rn) in period 6 have their valence electrons in the sixth energy level (n=6). The period number corresponds to the principal quantum number of the valence electrons.
Conclusion
The periodic table’s organization into families and periods is not merely a convenient arrangement; it reflects the fundamental relationship between electron configuration and chemical properties. Elements within the same family share similar valence electron configurations, leading to comparable chemical behaviors. Understanding these family trends and the concept of periods is crucial for grasping the systematic nature of chemistry and predicting the properties of elements.
Vocabulary
- Group (family): A vertical column in the periodic table, elements within a group share similar chemical properties.
- Alkali metals: Group 1A of the periodic table, highly reactive metals with one valence electron.
- Alkaline earth metals: Group 2A of the periodic table, reactive metals with two valence electrons.
- Halogens: Group 7A (or 17) of the periodic table, reactive nonmetals with seven valence electrons.
- Noble gases: Group 8A (or 18) of the periodic table, inert gases with a full outer shell of valence electrons.
- Transition elements: Groups 3 to 12 of the periodic table, metals with variable properties and multiple oxidation states.
- Period: A horizontal row in the periodic table, elements within a period have valence electrons in the same principal energy level.

